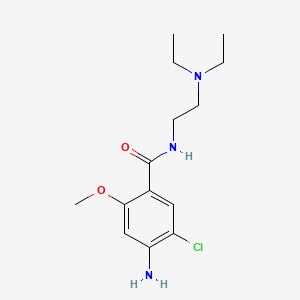

1. 4-amino-5-chloro-n-(2-(diethylamino)ethyl)-2-methoxybenzamide
2. Cerucal
3. Dihydrochloride, Metoclopramide
4. Hydrochloride, Metoclopramide
5. Maxolon
6. Metaclopramide
7. Metoclopramide Dihydrochloride
8. Metoclopramide Hydrochloride
9. Metoclopramide Monohydrochloride
10. Metoclopramide Monohydrochloride, Monohydrate
11. Monohydrochloride, Metoclopramide
12. Primperan
13. Reglan
14. Rimetin
1. 364-62-5
2. Methochlopramide
3. Metochlopramide
4. Metaclopramide
5. Primperan
6. Reliveran
7. Reglan
8. Metaclopromide
9. Metoclol
10. Moriperan
11. Elieten
12. Plasil
13. 4-amino-5-chloro-n-[2-(diethylamino)ethyl]-2-methoxybenzamide
14. Terperan
15. Maxolon
16. 2-methoxy-5-chloroprocainamide
17. 4-amino-5-chloro-n-(2-(diethylamino)ethyl)-2-methoxybenzamide
18. Methoclopramide
19. Metoclopramida
20. Metoclopramidum
21. Plasil (pharmaceutical)
22. Metoclopramidum [inn-latin]
23. Metoclopramida [inn-spanish]
24. 5-chloro-2-methoxyprocainamide
25. Clopra
26. Gastromax
27. Maxeran
28. Metocobil
29. Metramid
30. Parmid
31. Del 1267
32. 4-amino-5-chloro-n-(2-(diethylamino)ethyl)-o-anisamide
33. Methoxyclopramide
34. Benzamide, 4-amino-5-chloro-n-[2-(diethylamino)ethyl]-2-methoxy-
35. Metadrate
36. 4-amino-5-chloro-2-methoxy-n-(beta-diethylaminoethyl)benzamide
37. Clopra-"yellow"
38. N-(diethylaminoethyl)-2-methoxy-4-amino-5-chlorobenzamide
39. Benzamide, 4-amino-5-chloro-n-(2-(diethylamino)ethyl)-2-methoxy-
40. L4yeb44i46
41. Chebi:107736
42. 2-methoxy-4-amino-5-chloro-n,n-dimethylaminoethyl)benzamide
43. Ncgc00015643-07
44. 2-methoxy-4-amino-5-chloro-n,n-(dimethylaminoethyl)benzamide
45. O-anisamide, 4-amino-5-chloro-n-[2-(diethylamino)ethyl]-
46. Dsstox_cid_25169
47. Dsstox_rid_80719
48. Dsstox_gsid_45169
49. O-anisamide, 4-amino-5-chloro-n-(2-(diethylamino)ethyl)-
50. Megaldrate
51. Metoclopramide [inn:ban:jan]
52. Terperan (tn)
53. Cas-364-62-5
54. Elieten (tn)
55. Einecs 206-662-9
56. Brn 1884366
57. Unii-l4yeb44i46
58. Gimoli
59. Regla
60. Metochloropramide
61. Hsdb 7841
62. Reglan (salt/mix)
63. Maxolon (salt/mix)
64. Paspertin (salt/mix)
65. Spectrum_001638
66. 4-amino-5-chloro-n-(2-diethylaminoethyl)-2-methoxybenzamide
67. Chembl86
68. Prestwick0_000209
69. Prestwick1_000209
70. Prestwick2_000209
71. Prestwick3_000209
72. Spectrum2_001720
73. Spectrum3_000504
74. Spectrum4_000964
75. Spectrum5_001449
76. Lopac-m-0763
77. Biomol-nt_000082
78. Bmse000779
79. Metoclopramide [mi]
80. Metoclopramide [inn]
81. Metoclopramide [jan]
82. Lopac0_000762
83. Schembl18629
84. Bspbio_000197
85. Bspbio_002027
86. Gtpl241
87. Kbiogr_001307
88. Kbioss_002118
89. Metoclopramide (jp17/inn)
90. Metoclopramide [hsdb]
91. Cid_23659
92. Divk1c_000069
93. Metoclopramide [vandf]
94. Spbio_001740
95. Spbio_002118
96. Metoclopramide [mart.]
97. Us9132134, Metoclopramide
98. Bpbio1_000217
99. Bpbio1_001100
100. Metoclopramide [who-dd]
101. Dtxsid6045169
102. Primperan (tablet) (salt/mix)
103. Bdbm48320
104. Kbio1_000069
105. Kbio2_002118
106. Kbio2_004686
107. Kbio2_007254
108. Kbio3_001527
109. Amy8975
110. Ninds_000069
111. Hms2089g16
112. Hms3886p05
113. 4-amino-5-chloro-n-(2-diethylamino-ethyl)-2-methoxy-benzamide
114. Bcp30761
115. Gimoti (metoclopramide Nasal Spray)
116. Zinc1530716
117. Tox21_110188
118. 4-amino-5-chloro-n-(2-diethylamino-ethyl)-2-methoxy-benzamide (mcp)
119. Metoclopramide [ep Monograph]
120. Mfcd00211338
121. S5862
122. Stl257058
123. (metaclopramide)4-amino-5-chloro-n-(2-diethylamino-ethyl)-2-methoxy-benzamide
124. (metoclopramide)4-amino-5-chloro-n-(2-diethylamino-ethyl)-2-methoxy-benzamide
125. 4-amino-5-chloro-n-(2-diethylamino-ethyl)-2-methoxy-benzamide(metoclopramide)
126. Akos000280832
127. Tox21_110188_1
128. Ccg-204847
129. Cs-1064
130. Db01233
131. Ks-5055
132. Sdccgsbi-0050740.p005
133. 4-amino-5-chloro-n-(2-diethylamino-ethyl)-2-methoxy-benzamide (metoclopramide)
134. Idi1_000069
135. Ncgc00015643-01
136. Ncgc00015643-02
137. Ncgc00015643-03
138. Ncgc00015643-04
139. Ncgc00015643-05
140. Ncgc00015643-06
141. Ncgc00015643-08
142. Ncgc00015643-09
143. Ncgc00015643-11
144. Ncgc00015643-13
145. Ncgc00015643-18
146. Ncgc00024440-03
147. Ncgc00024440-04
148. Hy-17382
149. Nci60_003185
150. Cas-7232-21-5
151. Sbi-0050740.p004
152. Ab00053498
153. Ft-0628931
154. M2218
155. C07868
156. D00726
157. Ab00053498-13
158. Ab00053498_14
159. 232m215
160. 364m625
161. A823256
162. L001078
163. Metoclopramide, Vetranal(tm), Analytical Standard
164. Q421095
165. Brd-k75641298-001-01-5
166. Brd-k75641298-003-05-2
167. Brd-k75641298-003-16-9
168. Z2196779550
169. 2-methoxy-4-amino-5-chloro-n,n-dimethylaminoethylbenzamide
170. Metoclopramide, European Pharmacopoeia (ep) Reference Standard
171. N-(2-diethylaminoethyl)-2-methoxy-4-amino-5-chlorobenzamide
172. 4-amino-5-chloro-2-methoxy-n-(.beta.-diethylaminoethyl)benzamide
173. 4-amino-5-chloro-n-[2-(diethylamino)-ethyl]-2-methoxybenzamide
174. 4-amino-5-chloro-n-[2-(diethylamino)ethyl]-2-methoxy-benzamide;hydrochloride
175. 4-azanyl-5-chloranyl-n-[2-(diethylamino)ethyl]-2-methoxy-benzamide;hydrochloride
| Molecular Weight | 299.79 g/mol |
|---|---|
| Molecular Formula | C14H22ClN3O2 |
| XLogP3 | 2.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 7 |
| Exact Mass | 299.1400546 g/mol |
| Monoisotopic Mass | 299.1400546 g/mol |
| Topological Polar Surface Area | 67.6 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 300 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Metoclopramide |
| PubMed Health | Metoclopramide |
| Drug Classes | Antiemetic, Diagnostic Agent, Stimulant, Gastrointestinal |
| Drug Label | Metoclopramide hydrochloride is a white or practically white, crystalline, odorless or practically odorless powder. It is very soluble in water, freely soluble in alcohol, sparingly soluble in chloroform, practically insoluble in ether. Chemically it... |
| Active Ingredient | Metoclopramide hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 5mg base/ml |
| Market Status | Prescription |
| Company | Emcure Pharms |
| 2 of 4 | |
|---|---|
| Drug Name | Reglan |
| PubMed Health | Metoclopramide |
| Drug Classes | Antiemetic, Diagnostic Agent, Stimulant, Gastrointestinal |
| Drug Label | For oral administration, reglan tablets (metoclopramide tablets, USP) 10 mg are white, scored, capsule-shaped tablets engraved REGLAN on one side and ANI 10 on the opposite side.Each tablet contains:Metoclopramide base .................... |
| Active Ingredient | Metoclopramide hydrochloride |
| Dosage Form | Tablet; Injectable |
| Route | Injection; Oral |
| Strength | eq 5mg base; eq 5mg base/ml; eq 10mg base |
| Market Status | Prescription |
| Company | Ani Pharms; Baxter Hlthcare |
| 3 of 4 | |
|---|---|
| Drug Name | Metoclopramide |
| PubMed Health | Metoclopramide |
| Drug Classes | Antiemetic, Diagnostic Agent, Stimulant, Gastrointestinal |
| Drug Label | Metoclopramide hydrochloride is a white or practically white, crystalline, odorless or practically odorless powder. It is very soluble in water, freely soluble in alcohol, sparingly soluble in chloroform, practically insoluble in ether. Chemically it... |
| Active Ingredient | Metoclopramide hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 5mg base/ml |
| Market Status | Prescription |
| Company | Emcure Pharms |
| 4 of 4 | |
|---|---|
| Drug Name | Reglan |
| PubMed Health | Metoclopramide |
| Drug Classes | Antiemetic, Diagnostic Agent, Stimulant, Gastrointestinal |
| Drug Label | For oral administration, reglan tablets (metoclopramide tablets, USP) 10 mg are white, scored, capsule-shaped tablets engraved REGLAN on one side and ANI 10 on the opposite side.Each tablet contains:Metoclopramide base .................... |
| Active Ingredient | Metoclopramide hydrochloride |
| Dosage Form | Tablet; Injectable |
| Route | Injection; Oral |
| Strength | eq 5mg base; eq 5mg base/ml; eq 10mg base |
| Market Status | Prescription |
| Company | Ani Pharms; Baxter Hlthcare |
Antiemetics; Dopamine Antagonists
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
Metoclopramide tablets are indicated as short-term (4 to 12 weeks) therapy for adults with symptomatic, documented gastroesophageal reflux who fail to respond to conventional therapy. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for METOCLOPRAMIDE (metoclopramide hydrochloride) tablet (February 2010). Available from, as of October 12, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=15430
Metoclopramide tablets, USP is indicated for the relief of symptoms associated with acute and recurrent diabetic gastric stasis. The usual manifestations of delayed gastric emptying (eg, nausea, vomiting, heartburn, persistent fullness after meals, and anorexia) appear to respond to Metoclopramide Tablets within different time intervals. Significant relief of nausea occurs early and continues to improve over a three-week period. Relief of vomiting and anorexia may precede the relief of abdominal fullness by one week or more. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for METOCLOPRAMIDE (metoclopramide hydrochloride) tablet (February 2010). Available from, as of October 12, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=15430
Metoclopramide injection is indicated for the prophylaxis of vomiting associated with emetogenic cancer chemotherapy. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for METOCLOPRAMIDE (metoclopramide hydrochloride) injection, solution (July 2010). Available from, as of October 12, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=21962
For more Therapeutic Uses (Complete) data for Metoclopramide (8 total), please visit the HSDB record page.
WARNING: TARDIVE DYSKINESIA-Treatment with metoclopramide can cause tardive dyskinesia, a serious movement disorder that is often irreversible. The risk of developing tardive dyskinesia increases with duration of treatment and total cumulative dose. Metoclopramide therapy should be discontinued in patients who develop signs or symptoms of tardive dyskinesia. There is no known treatment for tardive dyskinesia. In some patients, symptoms may lessen or resolve after metoclopramide treatment is stopped. Treatment with metoclopramide for longer than 12 weeks should be avoided in all but rare cases where therapeutic benefit is thought to outweigh the risk of developing tardive dyskinesia.
US Natl Inst Health; DailyMed. Current Medication Information for METOCLOPRAMIDE (metoclopramide hydrochloride) tablet (February 2010). Available from, as of October 12, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=15430
Adverse reactions to metoclopramide generally involve the CNS and GI tract and are usually mild, transient, and reversible following discontinuance of the drug. In general, the incidence of metoclopramide-induced adverse effects is related to dosage and duration of therapy.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3024
The most frequent adverse effects of metoclopramide involve the CNS. Restlessness, drowsiness, fatigue, and lassitude have been reported in patients receiving the drug; these effects occur in about 10% of patients receiving a dosage of 10 mg 4 times daily. Insomnia, headache, confusion, dizziness, or depression with suicidal ideation occurs less frequently. The risk of drowsiness is increased at higher doses, occurring in about 70% of patients receiving doses of 1-2 mg/kg. Seizures have been reported rarely, although a causal relationship to metoclopramide has not been established. Hallucinations also have been reported rarely. Feelings of anxiety or agitation also may occur, especially following rapid IV injection of the drug.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3024
Extrapyramidal reactions (eg, acute dystonic reactions, akathisia) may occur in patients receiving metoclopramide and apparently are mediated via blockade of central dopaminergic receptors involved in motor function. Although extrapyramidal reactions may occur in all age groups and at any dose, they occur more frequently in pediatric patients and adults younger than 30 years of age and following IV administration of high doses of the drug (eg, those used in prophylaxis of cancer chemotherapy-induced vomiting). Extrapyramidal reactions generally occur within 24-48 hours after starting therapy and usually subside within 24 hours following discontinuance of the drug.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3024
For more Drug Warnings (Complete) data for Metoclopramide (31 total), please visit the HSDB record page.
Metoclopramide in the oral tablet form is used for symptomatic treatment of both acute and recurrent diabetic gastroparesis, in addition to the treatment of gastroesophageal reflux disease (GERD) in patients who have failed to respond to traditional therapy. A nasal spray formulation is also indicated to treat adults with acute, recurrent diabetic gastroparesis. In the intravenous injection form, it is indicated for the above conditions as well as for the prevention of vomiting that may follow emetogenic chemotherapy or nausea and vomiting after surgery. Intravenous metoclopramide facilitates intubation of the small bowel and stimulates gastric emptying and barium flow in patients who require radiological examination of the stomach or small intestine. In some cases, the delay of gastrointestinal emptying interferes with the radiographic visualization of the gastrointestinal tract, and metoclopramide is used to facilitate emptying in these cases, allowing for adequate diagnostic visualization. Some off-label uses of metoclopramide include the management of radiation-induced nausea and vomiting, gastric bezoars, intractable hiccups, and migraine pain.
FDA Label
Metoclopramide increases gastric emptying by decreasing lower esophageal sphincter (LES) pressure. It also exerts effects on the area postrema of the brain, preventing and relieving the symptoms of nausea and vomiting. In addition, this drug increases gastrointestinal motility without increasing biliary, gastric, or pancreatic secretions. Because of its antidopaminergic activity, metoclopramide can cause symptoms of tardive dyskinesia (TD), dystonia, and akathisia, and should therefore not be administered for longer than 12 weeks.
Antiemetics
Drugs used to prevent NAUSEA or VOMITING. (See all compounds classified as Antiemetics.)
Dopamine D2 Receptor Antagonists
Compounds and drugs that bind to and inhibit or block the activation of DOPAMINE D2 RECEPTORS. (See all compounds classified as Dopamine D2 Receptor Antagonists.)
A03FA01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A03 - Drugs for functional gastrointestinal disorders
A03F - Propulsives
A03FA - Propulsives
A03FA01 - Metoclopramide
Absorption
Metoclopramide is rapidly absorbed in the gastrointestinal tract with an absorption rate of about 84%. The bioavailability of the oral preparation is reported to be about 40.7%, but can range from 30-100%. Nasal metoclopramide is 47% bioavailable. A 15mg dose reaches a Cmax of 41.0 ng/mL, with a Tmax of 1.25 h, and an AUC of 367 ng\*h/mL.
Route of Elimination
About 85% of an orally administered dose was measured in the urine within 72 hours during a pharmacokinetic study. An average of 18% to 22% of 10-20 mg dose was recovered as free drug within 3 days of administration.
Volume of Distribution
The volume of distribution of metoclopramide is approximately 3.5 L/kg. This implies a high level of tissue distribution. Metoclopramide crosses the placental barrier and can cause extrapyramidal symptoms in the fetus.
Clearance
The renal clearance of metoclopramide is 0.16 L/h/kg with a total clearance of 0.7 L/h/kg. Clinical studies showed that the clearance of metoclopramide may be reduced by up to 50% in patients with renal impairment. After high intravenous doses, total metoclopramide clearance ranged from 0.31 to 0.69 L/kg/h.
Metoclopramide is rapidly and almost completely absorbed from the GI tract following oral administration; however, absorption may be delayed or diminished in patients with gastric stasis. Considerable interindividual variations (up to fivefold) in peak plasma concentration have been reported with the same oral dose of metoclopramide. This variability apparently results from interindividual differences in first-pass metabolism of the drug.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3027
Bioavailability of metoclopramide appears to correlate with the ratio of free:conjugated metoclopramide concentrations in urine. It appears that sulfate conjugation in the GI lumen and/or during first pass through the liver is the principal determinant of bioavailability of orally administered metoclopramide. The absolute bioavailability of orally administered metoclopramide has not been clearly established in humans, but limited data indicate that 30-100% of an oral dose of the drug reaches systemic circulation as unchanged metoclopramide. Following IM administration, the absolute bioavailability of metoclopramide is 74-96%.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3027
Following oral administration of a single 10-mg dose of the drug in healthy, fasting adults in one study, peak plasma metoclopramide concentrations of 32-44 ng/mL occurred at 1-2 hours; following oral administration of a single 20-mg dose, peak plasma metoclopramide concentrations of 72-87 ng/mL occurred at an average of 2 hours.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3027
In a study in infants (3.5 weeks-5.4 months of age) with gastroesophageal reflux who received 0.15-mg/kg oral doses of metoclopramide every 6 hours for 10 doses as an oral solution, the mean peak plasma concentration (56.8 ng/mL) of the drug after the 10th dose was twofold higher compared with that after the first dose (29 ng/mL), suggesting that metoclopramide accumulates in plasma following multiple oral dosing in this age group. In these patients, time to reach mean peak plasma concentrations (2.2 hours) was similar after the 10th dose to that occurring after the first dose.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3027
For more Absorption, Distribution and Excretion (Complete) data for Metoclopramide (18 total), please visit the HSDB record page.
Metoclopramide undergoes first-pass metabolism and its metabolism varies according to the individual. This drug is metabolized by cytochrome P450 enzymes in the liver. CYP2D6 and CYP3A4 both contribute to its metabolism, with CYP2D6 being more heavily involved. CYP1A2 is also a minor contributing enzyme. The process of N-4 sulphate conjugation is a primary metabolic pathway of metoclopramide.
Although the exact metabolic fate of metoclopramide is not clearly established, it appears that metoclopramide is only minimally metabolized. The major metabolite found in urine is 2-[(4-amino-5-chloro-2-methoxybenzoyl)amino]acetic acid; it is not known if this metabolite is pharmacologically active. Metoclopramide is conjugated with sulfuric and/or glucuronic acid.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3028
Metoclopramide has known human metabolites that include monodeethylmetoclopramide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The mean elimination half-life of metoclopramide in people with healthy renal function ranges from 5 to 6 hours but is prolonged in patients with renal impairment. Downward dose adjustment should be considered.
In adults, the half-life of metoclopramide in the initial phase (t1/2 alpha) is about 5 minutes, and the half-life in the terminal phase (t1/2 beta) ranges from 2.5-6 hours. In children receiving oral or IV metoclopramide, the elimination half-life of the drug reportedly is 4.1-4.5 hours. Following oral administration of 0.15-mg/kg doses of metoclopramide every 6 hours for 10 doses in an infant (3.5 weeks of age), elimination half-lives of 23.1 and 10.3 hours were observed after the first and 10th dose, respectively, which were substantially longer than those reported in older infants, suggesting a reduced clearance in the neonate possibly being associated with immature renal and hepatic functions present at birth.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3027
Metoclopramide causes antiemetic effects by inhibiting dopamine D2 and serotonin 5-HT3 receptors in the chemoreceptor trigger zone (CTZ) located in the area postrema of the brain. Administration of this drug leads to prokinetic effects via inhibitory actions on presynaptic and postsynaptic D2 receptors, agonism of serotonin 5-HT4 receptors, and antagonism of muscarinic receptor inhibition. This action enhances the release of acetylcholine, causing increased lower esophageal sphincter (LES) and gastric tone, accelerating gastric emptying and transit through the gut. Metoclopramide antagonizes the dopamine D2 receptors. Dopamine exerts relaxant effect on the gastrointestinal tract through binding to muscular D2 receptors.
Metoclopramide accelerates gastric emptying and intestinal transit from the duodenum to the ileocecal valve by increasing the amplitude and duration of esophageal contractions, the resting tone of the lower esophageal sphincter, and the amplitude and tone of gastric (especially antral) contractions and by relaxing the pyloric sphincter and the duodenal bulb, while increasing peristalsis of the duodenum and jejunum. Unlike nonspecific cholinergic-like stimulation of upper GI smooth muscle, the stimulant effects of metoclopramide on GI smooth muscle coordinate gastric, pyloric, and duodenal motor activity.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3026
The pharmacologic actions of metoclopramide on the upper GI tract are similar to those of cholinergic drugs (e.g., bethanechol); however, unlike cholinergic drugs, metoclopramide does not stimulate gastric, biliary, or pancreatic secretions and does not affect serum gastrin concentration. Although the exact mechanism of action of metoclopramide is unclear, the effects of metoclopramide on GI motility may be mediated via enhancement of cholinergic excitatory processes at the postganglionic neuromuscular junction; antagonism of nonadrenergic, noncholinergic inhibitory motor nerves (i.e., dopaminergic); and/or a direct effect on smooth muscle.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3026
The effects of metoclopramide on GI motility do not depend on intact vagal innervations but are reduced or abolished by anticholinergic drugs (e.g., atropine) and potentiated by cholinergic drugs (e.g., carbachol, methacholine). These findings suggest that metoclopramide's effects on GI motility may depend in part on intramural cholinergic neurons of smooth muscle that are intact after vagal denervation. Unlike cholinergic drugs, metoclopramide requires intrinsic neuronal storage sites of acetylcholine to exert its pharmacologic effects. Postsynaptic activity results from metoclopramide's ability to enhance release of acetylcholine from postganglionic cholinergic neurons in the GI tract and to sensitize muscarinic receptors of GI smooth muscle to the actions of acetylcholine.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3026
Metoclopramide is a potent dopamine-receptor antagonist, and some of the actions of metoclopramide on GI smooth muscle may be mediated via antagonism of dopaminergic neurotransmission, Specific dopamine receptors in the esophagus and stomach have been identified; however, it is not known if there is a dopaminergic control system for smooth muscle function in the upper GI tract. In the GI tract, dopamine is principally an inhibitory neurotransmitter. Dopamine decreases the intensity of esophageal contractions, relaxes the proximal stomach, and reduces gastric secretion. Although metoclopramide blocks these inhibitory effects of dopamine, the actual role of dopamine in the peripheral control of GI motility has not been fully elucidated. Since cholinergic mechanisms are responsible for most excitatory motor activity in the GI tract, it appears that metoclopramide's therapeutic effects are principally caused by the drug's cholinergic-like activity; however, antagonism of GI dopaminergic activity may augment metoclopramide's cholinergic-like activity.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3027
For more Mechanism of Action (Complete) data for Metoclopramide (10 total), please visit the HSDB record page.