


1. Boro Scopol
2. Boro-scopol
3. Hyoscine
4. Isopto Hyoscine
5. Kwells
6. Scoburen
7. Scopace
8. Scopoderm Tts
9. Scopolamine
10. Scopolamine Cooper
11. Transderm Scop
12. Transderm V
13. Transderm-v
14. Travacalm Ho
15. Vorigeno
1. 114-49-8
2. Scopolamine Hbr
3. Hyoscine Hydrobromide
4. Scopos
5. (-)-scopolamine Hydrobromide
6. Scopolamine (hydrobromide)
7. Beldavrin
8. Scopamin
9. Tranaxine
10. Hyosol
11. Hyoscine Bromide
12. (-)-hyoscine Hydrobromide
13. Scopolaminium Bromide
14. Scopolammonium Bromide
15. Euscopol
16. Isoscopil
17. Hysco
18. Kwells
19. (-)-scopolamine Bromide
20. Scopolamine Bromide
21. Scopolamine Hydrobromide Anhydrous
22. R3k67drl3j
23. L-hyoscine Hydrobromide
24. Sereen
25. Mls000069527
26. L-scopolamine-hydrobromide
27. Chebi:61271
28. Smr000058594
29. Dsstox_cid_12029
30. Dsstox_rid_78902
31. Dsstox_gsid_32029
32. Cas-114-49-8
33. Unii-r3k67drl3j
34. Einecs 204-050-6
35. Nsc 61806
36. Nsc-61806
37. Opera_id_496
38. Ec 204-050-6
39. Hyoscine Bromide (anhydrous)
40. Schembl40627
41. Scopolamine Bromide (anhydrous)
42. Chembl3185877
43. Dtxsid0032029
44. Hy-n0296a
45. Scopolaminium Bromide (anhydrous)
46. Regid_for_cid_6603108
47. Scopolammonium Bromide (anhydrous)
48. 672-21-9
49. Scopolamine Hydrobromide (anhydrous)
50. Tox21_110035
51. Tox21_302019
52. (-)-scopolamine Bromide (anhydrous)
53. S2508
54. Scopolamine Hydrobromide [mi]
55. (-)-hyoscine Hydrobromide (anhydrous)
56. Akos015965267
57. Hyoscine Hydrobromide [who-dd]
58. Tox21_110035_1
59. Ac-3389
60. Ccg-268451
61. Cs-2000
62. 1alphah,5alphah-tropan-3-alpha-ol, 5beta,7beta-epoxy-, (-)-tropate (ester), Hydrobromide
63. Ncgc00013722-01
64. Ncgc00024357-07
65. Ncgc00255166-01
66. (-)-scopolamine Hydrobromide (anhydrous)
67. As-35324
68. N2570
69. Sw199343-2
70. 114s498
71. A935283
72. Sr-01000597758
73. Sr-01000597758-1
74. W-108599
75. Q27130957
76. (-)-scopolamine Hydrobromide;hyoscine Hydrobromide;scopine Hydrobromide
77. 1alphah,5alphah-tropan-3alpha-ol, 6beta,7beta-epoxy-, (-)-tropate (ester), Hydrobromide
78. (?,s)-?-(hydroxymethyl)benzeneacetic Acid (1?,2?,4?,5?,7?)-9-methyl-3-oxa-9-azatricyclo[3.3.1.02,4]non-7-yl Ester Hydrobromide
79. (1r,2r,4s,5s,7s)-9-methyl-3-oxa-9-azatricyclo[3.3.1.0(2,4)]non-7-yl (2s)-3-hydroxy-2-phenylpropanoate Hydrobromide
80. (1r,2r,4s,5s,7s,9s)-7-{[(2s)-3-hydroxy-2-phenylpropanoyl]oxy}-9-methyl-3-oxa-9-azoniatricyclo[3.3.1.0(2,4)]nonane Bromide
81. 1-alpha-h,5-alpha-h-tropan-3-alpha-ol, 6-beta,7-beta-epoxy-, (-)-tropate (ester), Hydrobromide
82. 6.beta.,7.beta.-epoxy-1.alpha.h,5.alpha.h-tropan-3.alpha.-ol (-)-tropate (ester) Hydrobromide
83. Benzeneacetic Acid, .alpha.-(hydroxymethyl)-, (1.alpha.,2.beta.,4.beta.,5.alpha.,7.beta.)-9-methyl-3-oxa-9-azatricyclo3.3.1.02,4non-7-yl Ester, (.alpha.s)- Hydrobromide
84. Benzeneacetic Acid, Alpha-(hydroxymethyl)-, (1alpha,2beta,4beta,5alpha,7beta)-9-methyl-3-oxa-9-azatricyclo(3.3.1.02,4)non-7-yl Ester, Hydrobromide (1:1), (alphas)-
85. Benzeneacetic Acid, Alpha-(hydroxymethyl)-, (1alpha,2beta,4beta,5alpha,7beta)-9-methyl-3-oxa-9-azatricyclo(3.3.1.02,4)non-7-yl Ester, Hydrobromide, (alphas)-
86. Benzeneacetic Acid, Alpha-(hydroxymethyl)-, 9-methyl-3-oxa-9-azatricyclo(3.3.1.02,4)non-7-yl Ester, Hydrobromide, (7(s)-(1alpha,2beta,4beta,5alpha,7beta))-
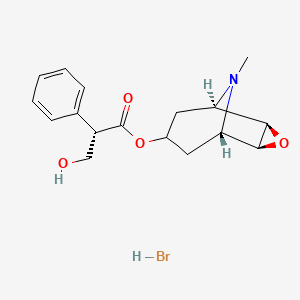
| Molecular Weight | 384.3 g/mol |
|---|---|
| Molecular Formula | C17H22BrNO4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 5 |
| Exact Mass | 383.07322 g/mol |
| Monoisotopic Mass | 383.07322 g/mol |
| Topological Polar Surface Area | 62.3 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 418 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Adjuvants, Anesthesia
Agents that are administered in association with anesthetics to increase effectiveness, improve delivery, or decrease required dosage. (See all compounds classified as Adjuvants, Anesthesia.)
Antiemetics
Drugs used to prevent NAUSEA or VOMITING. (See all compounds classified as Antiemetics.)
Cholinergic Antagonists
Drugs that bind to but do not activate CHOLINERGIC RECEPTORS, thereby blocking the actions of ACETYLCHOLINE or cholinergic agonists. (See all compounds classified as Cholinergic Antagonists.)
Muscarinic Antagonists
Drugs that bind to but do not activate MUSCARINIC RECEPTORS, thereby blocking the actions of endogenous ACETYLCHOLINE or exogenous agonists. Muscarinic antagonists have widespread effects including actions on the iris and ciliary muscle of the eye, the heart and blood vessels, secretions of the respiratory tract, GI system, and salivary glands, GI motility, urinary bladder tone, and the central nervous system. (See all compounds classified as Muscarinic Antagonists.)
Mydriatics
Agents that dilate the pupil. They may be either sympathomimetics or parasympatholytics. (See all compounds classified as Mydriatics.)
