


1. Gadolinium Texaphyrin
2. Gadolinium Texaphyrin Complex
3. Gd(iii) Texaphyrin
4. Motexafin Gadolinium
5. Pci-0120
6. Xcytrin
1. Motexafin Gadolinium
2. Xcytrin
3. Gadolinium Texaphyrin
4. 246252-06-2
5. 156436-89-4
6. Pci-0120
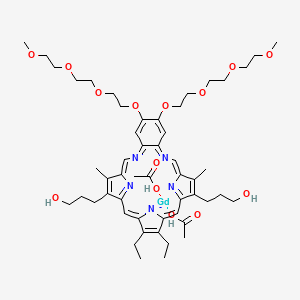
7. Acetic Acid;3-[4,5-diethyl-24-(3-hydroxypropyl)-16,17-bis[2-[2-(2-methoxyethoxy)ethoxy]ethoxy]-10,23-dimethyl-13,20,25,26-tetraza-27-azanidapentacyclo[20.2.1.13,6.18,11.014,19]heptacosa-1(25),2,4,6,8(26),9,11,13,15,17,19,21,23-tridecaen-9-yl]propan-1-ol;gadolinium
8. Api-gp 3; Gd-tex; Gadolinium Texaphyrin; Gd Texaphyrin; Pci 0120; Xcytrin
9. 3-[4,5-diethyl-24-(3-hydroxypropyl)-16,17-bis[2-[2-(2-methoxyethoxy)ethoxy]ethoxy]-10,23-dimethyl-13,20,25,26-tetraza-27-azanidapentacyclo[20.2.1.13,6.18,11.014,19]heptacosa-1(25),2,4,6,8(26),9,11,13,15,17,19,21,23-tridecaen-9-yl]propan-1-ol;gadolinium(3+);diacetate
10. Gadolinium Diacetate (1z,7z,12e,20e)-4,5-diethyl-9,24-bis(3-hydroxypropyl)-16,17-bis{2-[2-(2-methoxyethoxy)ethoxy]ethoxy}-10,23-dimethyl-13,20,25,26,27-pentaazapentacyclo[20.2.1.13,6.18,11.014,19]heptacosa-1,3,5,7,9,11(26),12,14,16,18,20,22(25),23-tridecaen-27-ide
11. Gdt2b2
12. Mgd
13. Dtxsid4037083
| Molecular Weight | 1150.4 g/mol |
|---|---|
| Molecular Formula | C52H74GdN5O14- |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 19 |
| Rotatable Bond Count | 28 |
| Exact Mass | 1150.44734 g/mol |
| Monoisotopic Mass | 1150.44734 g/mol |
| Topological Polar Surface Area | 239 Ų |
| Heavy Atom Count | 72 |
| Formal Charge | -1 |
| Complexity | 2080 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 4 |
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Contrast Media
Substances used to allow enhanced visualization of tissues. (See all compounds classified as Contrast Media.)
Photosensitizing Agents
Drugs that are pharmacologically inactive but when exposed to ultraviolet radiation or sunlight are converted to their active metabolite to produce a beneficial reaction affecting the diseased tissue. These compounds can be administered topically or systemically and have been used therapeutically to treat psoriasis and various types of neoplasms. (See all compounds classified as Photosensitizing Agents.)
