





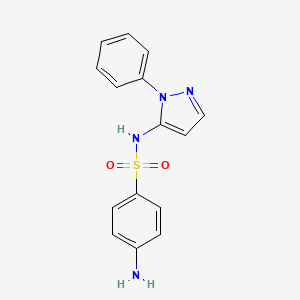

1. Phenylsulfapyrazole
2. Sulfaphenylpyrazol
3. Sulphaphenazole
1. 526-08-9
2. Sulphaphenazole
3. Sulfabid
4. 4-amino-n-(1-phenyl-1h-pyrazol-5-yl)benzenesulfonamide
5. Plisulfan
6. Raziosulfa
7. Sulfafenazol
8. Sulfaphenazol
9. Sulfaphenazon
10. Depocid
11. Depotsulfonamide
12. Sulphenazole
13. Sulfaphenylpyrazole
14. Sulfaphenylpipazol
15. Sulfaphenazolum
16. Merian
17. Sulfafenazolo
18. Eftolon
19. Firmazolo
20. Paidazolo
21. Inamil
22. Isarol
23. Orisul
24. 1-phenyl-5-sulfanilamidopyrazole
25. 5-sulfanilamido-1-phenylpyrazole
26. Microtan Pirazolo
27. N'-(1-phenylpyrazol-5-yl)sulfanilamide
28. Sulfafenazolo [italian]
29. 3-(p-aminobenzenesulfonamido)-2-phenylpyrazole
30. Sulfafenazol [inn-spanish]
31. Sulfaphenazol [inn-french]
32. Sulfaphenazolum [inn-latin]
33. Benzenesulfonamide, 4-amino-n-(1-phenyl-1h-pyrazol-5-yl)-
34. 4-amino-n-(2-phenylpyrazol-3-yl)benzenesulfonamide
35. N1-(1-phenylpyrazol-5-yl)sulfanilamide
36. N(sup 1)-(1-phenylpyrazol-5-yl)sulfanilamide
37. 4-amino-n-(1-phenyl-1h-pyrazol-5-yl)benzene-1-sulfonamide
38. Nsc-757859
39. Chembl1109
40. N(1)-(1-phenylpyrazol-5-yl)sulfanilamide
41. Chebi:77780
42. 0j8l4v3f81
43. Sulfaphenylpyrazol
44. Phenylsulfapyrazole
45. Ncgc00015925-02
46. Solfafenazolo
47. Cas-526-08-9
48. Solfafenazolo [dcit]
49. Dsstox_cid_24131
50. Dsstox_rid_80106
51. Dsstox_gsid_44131
52. Orisulf
53. Isarol V
54. Smr000326684
55. Prestwick_454
56. Unii-0j8l4v3f81
57. Sr-01000075649
58. Einecs 208-384-3
59. Lopac-s-0758
60. Sulfaphenazole (jan/inn)
61. Brn 0308518
62. Sulfonylpyrazol
63. Sulfaphenazole [inn:ban:jan]
64. Sulfabid (tn)
65. Sulfanilamide, N(sup 1)-(1-phenylpyrazol-5-yl)-
66. Mfcd00057226
67. Spectrum_001406
68. Prestwick0_000021
69. Prestwick1_000021
70. Prestwick2_000021
71. Prestwick3_000021
72. Spectrum2_001943
73. Spectrum3_001741
74. Spectrum4_000443
75. Spectrum5_001185
76. Sulfaphenazole, >=98%
77. Sulfanilamide, N1-(1-phenylpyrazol-5-yl)-
78. Sulfaphenazole [mi]
79. Sulfaphenazole [inn]
80. Sulfaphenazole [jan]
81. Lopac0_001095
82. Bspbio_000081
83. Bspbio_003442
84. Kbiogr_000826
85. Kbioss_001886
86. 5-25-09-00415 (beilstein Handbook Reference)
87. Mls000859612
88. Mls001056713
89. Divk1c_000303
90. Schembl122040
91. Spectrum1501143
92. Spbio_002002
93. Spbio_002005
94. Sulfaphenazole [mart.]
95. Bpbio1_000091
96. Sulfaphenazole [who-dd]
97. Dtxsid2044131
98. Gtpl11352
99. Hms500p05
100. Kbio1_000303
101. Kbio2_001886
102. Kbio2_004454
103. Kbio2_007022
104. Kbio3_002662
105. Zinc57490
106. 4-amino-n-(1-phenyl-1h-pyrazol-5-yl)-benzenesulfonamide
107. 4-amino-n-(2-phenyl-2h-pyrazol-3-yl)-benzenesulfonamide
108. Ninds_000303
109. Hms1568e03
110. Hms1921j13
111. Hms2095e03
112. Hms2235j21
113. Hms3263k12
114. Hms3373e16
115. Hms3712e03
116. Pharmakon1600-01501143
117. Sulfaphenazole, >=98% (hplc)
118. Albb-025160
119. Bcp27626
120. Hy-b1218
121. Str05485
122. Sulfaphenazole [orange Book]
123. Tox21_110262
124. Tox21_501095
125. Bdbm50090677
126. Ccg-39431
127. Nsc757859
128. Pyrazole, 1-phenyl-5-sulfanilamido-
129. S3673
130. Stk663863
131. Akos003348743
132. Tox21_110262_1
133. Cs-4843
134. Db06729
135. Lp01095
136. Nsc 757859
137. Sdccgsbi-0051065.p004
138. Idi1_000303
139. Ncgc00015925-01
140. Ncgc00015925-03
141. Ncgc00015925-04
142. Ncgc00015925-05
143. Ncgc00015925-06
144. Ncgc00015925-07
145. Ncgc00015925-08
146. Ncgc00015925-09
147. Ncgc00015925-10
148. Ncgc00015925-12
149. Ncgc00015925-13
150. Ncgc00015925-17
151. Ncgc00094368-01
152. Ncgc00094368-02
153. Ncgc00094368-03
154. Ncgc00094368-04
155. Ncgc00094368-05
156. Ncgc00261780-01
157. Sulfaphenazole 100 Microg/ml In Methanol
158. Sbi-0051065.p003
159. Ab00052218
160. Eu-0101095
161. Ft-0761575
162. Sulfaphenazole 100 Microg/ml In Acetonitrile
163. Bim-0051065.0001
164. D01954
165. D70410
166. S 0758
167. Ab00052218_10
168. Q6577293
169. Sr-01000075649-2
170. Sr-01000075649-7
171. Sr-01000075649-8
172. Sr-01000075649-9
173. Brd-k10671814-001-05-6
174. Brd-k10671814-001-09-8
175. 4-amino-n-(1-phenyl-1h-pyrazol-5-yl)-1-benzenesulfonamide
176. Brn-0308518; Brn0308518; Brn 0308518;sulfabid;sulfaphenylpyrazol;sulphaphenazole
| Molecular Weight | 314.4 g/mol |
|---|---|
| Molecular Formula | C15H14N4O2S |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 4 |
| Exact Mass | 314.08374688 g/mol |
| Monoisotopic Mass | 314.08374688 g/mol |
| Topological Polar Surface Area | 98.4 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 451 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment bacterial infections.
Anti-Infective Agents
Substances that prevent infectious agents or organisms from spreading or kill infectious agents in order to prevent the spread of infection. (See all compounds classified as Anti-Infective Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01E - Sulfonamides and trimethoprim
J01ED - Long-acting sulfonamides
J01ED08 - Sulfaphenazole
S - Sensory organs
S01 - Ophthalmologicals
S01A - Antiinfectives
S01AB - Sulfonamides
S01AB05 - Sulfafenazol
Hepatic.
Sulfaphenazole is a sulfonamide antibacterial. In bacteria, antibacterial sulfonamides act as competitive inhibitors of the enzyme dihydropteroate synthetase (DHPS), an enzyme involved in folate synthesis. As such, the microorganism will be "starved" of folate and die.
