







1. Biphenabid
2. Dh 581
3. Dh-581
4. Dh581
5. Lorelco
6. Lurselle
7. Panavir
8. Superlipid
1. 23288-49-5
2. Lorelco
3. Biphenabid
4. Bisphenabid
5. Lurselle
6. Bisbid
7. Lesterol
8. Lursell
9. Dh-581
10. Panavir
11. Probucolum
12. Sinlestal
13. Probucolum [inn-latin]
14. Dh 581
15. Acetone Bis(3,5-di-tert-butyl-4-hydroxyphenyl) Mercaptole
16. 4,4'-(isopropylidenedithio)bis(2,6-di-tert-butylphenol)
17. 2,6-ditert-butyl-4-[2-(3,5-ditert-butyl-4-hydroxyphenyl)sulfanylpropan-2-ylsulfanyl]phenol
18. 4,4'-(propane-2,2-diylbis(sulfanediyl))bis(2,6-di-tert-butylphenol)
19. Nsc 86225
20. Nsc 652160
21. Acetone, Bis(3,5-di-tert-butyl-4-hydroxyphenyl) Mercaptole
22. 2,6-di-tert-butyl-4-({2-[(3,5-di-tert-butyl-4-hydroxyphenyl)sulfanyl]propan-2-yl}sulfanyl)phenol
23. Nsc-86225
24. Chembl608
25. Nsc-652160
26. 4,4'-(propane-2,2-diyldisulfanediyl)bis(2,6-di-tert-butylphenol)
27. P3cth044xj
28. Mls000028492
29. Chebi:8427
30. Nsc86225
31. Phenol, 4,4'-[(1-methylethylidene)bis(thio)]bis[2,6-bis(1,1-dimethylethyl)-
32. Nsc652160
33. 4,4'-(isopropylidenedithio)bis(2, 6-di-tert-butylphenol)
34. 4,4'-(isopropylidenedithio)bis[2, 6-di-tert-butylphenol]
35. Ncgc00016777-01
36. Smr000058265
37. Superlipid
38. Cas-23288-49-5
39. Phenol, 4,4'-((1-methylethylidene)bis(thio))bis(2,6-bis(1,1-dimethylethyl)-
40. Dsstox_cid_25440
41. Dsstox_rid_80881
42. Dsstox_gsid_45440
43. 4,4'- (isopropylidenedithio)bis(2,6-di-tert-butylphenol)
44. Ccris 7510
45. Dh581
46. Sr-01000695447
47. Lorelco (tn)
48. Einecs 245-560-9
49. Unii-p3cth044xj
50. Brn 2026253
51. Serterol
52. Probucol,(s)
53. Prestwick_408
54. Mfcd00079281
55. Probucol [usan:usp:inn:ban:jan]
56. De-3872
57. Spectrum_001296
58. Acetone, Bis (3,5-di-tert-butyl-4-hydroxyphenyl) Mercaptole
59. Probucol [usan]
60. Probucol [inn]
61. Probucol [jan]
62. Probucol [mi]
63. Probucol [vandf]
64. Opera_id_1820
65. Prestwick0_000384
66. Prestwick1_000384
67. Prestwick2_000384
68. Prestwick3_000384
69. Spectrum2_001400
70. Spectrum3_001438
71. Spectrum4_000414
72. Spectrum5_001343
73. Probucol [mart.]
74. Probucol [usp-rs]
75. Probucol [who-dd]
76. Schembl4150
77. Bspbio_000567
78. Bspbio_003176
79. Kbiogr_000708
80. Kbiogr_002394
81. Kbioss_001776
82. Kbioss_002399
83. Probucol, Analytical Standard
84. Divk1c_000599
85. Spectrum1501109
86. Probucol (jp17/usp/inn)
87. Spbio_001420
88. Spbio_002488
89. Probucol [orange Book]
90. Bpbio1_000625
91. Gtpl7277
92. Probucol [usp Impurity]
93. Dtxsid2045440
94. Hms501n21
95. Kbio1_000599
96. Kbio2_001776
97. Kbio2_002394
98. Kbio2_004344
99. Kbio2_004962
100. Kbio2_006912
101. Kbio2_007530
102. Kbio3_002396
103. Kbio3_002873
104. Cmap_000039
105. Ninds_000599
106. 4,4'- (isopropylidenedithio)bis(2, 6-di-tert-butylphenol)
107. Hms1569m09
108. Hms1921d21
109. Hms2092d05
110. Hms2096m09
111. Hms2233c08
112. Hms3655p19
113. Hms3713m09
114. Pharmakon1600-01501109
115. Bcp06487
116. Hy-b0388
117. Zinc1530755
118. Tox21_110605
119. Bdbm50007260
120. Ccg-38986
121. Nsc757837
122. S2119
123. Stk762566
124. Akos001740866
125. Tox21_110605_1
126. Db01599
127. Ks-1459
128. Nsc-757837
129. 2,6-bis(tert-butyl)-4-{1-[3,5-bis(tert-butyl)-4-hydroxyphenylthio]-isopropylth Io}phenol
130. Idi1_000599
131. Ncgc00016777-02
132. Ncgc00016777-03
133. Ncgc00016777-04
134. Ncgc00016777-05
135. Ncgc00016777-06
136. Ncgc00016777-07
137. Ncgc00016777-08
138. Ncgc00094895-01
139. Ncgc00094895-02
140. Ncgc00094895-03
141. Ncgc00094895-04
142. Ac-31457
143. Bp166196
144. Sbi-0051640.p002
145. Db-046120
146. Ab00052202
147. Ft-0630498
148. P2002
149. Sw196648-3
150. C07373
151. D00476
152. F20384
153. Ab00052202_14
154. Ab00052202_15
155. 288p495
156. A918612
157. Acetone,5-di-tert-butyl-4-hydroxyphenyl) Mercaptole
158. Sr-01000695447-2
159. Sr-01000695447-3
160. W-107409
161. Brd-k72029282-001-04-8
162. Brd-k72029282-001-15-4
163. Q10354103
164. Acetone Bis(3,5-di-t-butyl-4-hydroxyphenyl) Mercaptole
165. Z1551900339
166. Bis(3,5-di-tert-butyl-4-hydroxyphenyl) Mercaptole Acetone
167. Probucol, United States Pharmacopeia (usp) Reference Standard
168. Phenol,4'-[(1-methylethylidene)bis(thio)]bis[2,6-bis(1,1-dimethylethyl)-
169. 2,6-di-tert-butyl-4-(2-(3,5-di-tert-butyl-4-hydroxyphenylthio)propan-2-ylthio)phenol
170. 4,4'-[(1-methylethylidene)bis(thio)]bis[2,6-bis(1,1-dimethylethyl)phenol]
171. 2,6-di(tert-butyl)-4-[(1-{[3,5-di(tert-butyl)-4-hydroxyphenyl]sulfanyl}-1-methylethyl)sulfanyl]phenol
172. 2,6-di(tert-butyl)-4-{1-[3,5-di(tert-butyl)-4-hydroxyphenylsulfanyl]-1-methylethylsulfanyl}phenol
173. 2,6-di(tert-butyl)-4-{1-[3,5-di(tert-butyl)-4-hydroxyphenylsulfanyl]-1-methylethylsulfanyl}phenol(probucol)
174. 2,6-di-tert-butyl-4-[1-(3,5-di-tert-butyl-4-hydroxy-phenylsulfanyl)-1-methyl-ethylsulfanyl]-phenol
175. 2,6-ditert-butyl-4-((1-[(3,5-ditert-butyl-4-hydroxyphenyl)sulfanyl]-1-methylethyl)sulfanyl)phenol #
176. 2,6-ditert-butyl-4-[1-(3,5-ditert-butyl-4-hydroxy-phenyl)sulfanyl-1-methyl-ethyl]sulfanyl-phenol
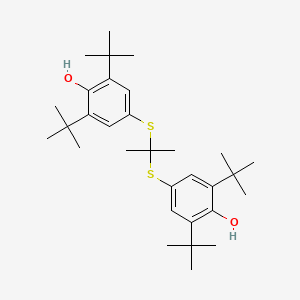
| Molecular Weight | 516.8 g/mol |
|---|---|
| Molecular Formula | C31H48O2S2 |
| XLogP3 | 11.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 8 |
| Exact Mass | 516.30957312 g/mol |
| Monoisotopic Mass | 516.30957312 g/mol |
| Topological Polar Surface Area | 91.1 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 0 |
| Complexity | 583 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Used to lower LDL and HDL cholesterol.
Probucol lowers cholesterol levels by increasing LDL (low-density lipoprotein) breakdown. Additionally, probucol may inhibit cholesterol synthesis and delay cholesterol absorption. Probucol is a powerful antioxidant drug normally used to prevent vascular disease caused by the free radicals in the body.
Anticholesteremic Agents
Substances used to lower plasma cholesterol levels. (See all compounds classified as Anticholesteremic Agents.)
Antioxidants
Naturally occurring or synthetic substances that inhibit or retard oxidation reactions. They counteract the damaging effects of oxidation in animal tissues. (See all compounds classified as Antioxidants.)
C - Cardiovascular system
C10 - Lipid modifying agents
C10A - Lipid modifying agents, plain
C10AX - Other lipid modifying agents
C10AX02 - Probucol
Absorption
Absorption from the gastrointestinal tract is limited and variable (about 7%).
Ranges from 12 hours to more than 500 hours, the longest half-life probably being in adipose tissue.
Probucol lowers serum cholesterol by increasing the fractional rate of low-density lipoprotein (LDL) catabolism in the final metabolic pathway for cholesterol elimination from the body. This drug may also act to inhibit the initial stages of cholesterol synthesis and act to inhibit the absorption of cholesterol from the diet. Recent information suggests that probucol may inhibit the oxidation and tissue deposition of LDL cholesterol, thereby inhibiting atherogenesis. It appears to inhibits ABCA1-mediated cellular lipid efflux.
