






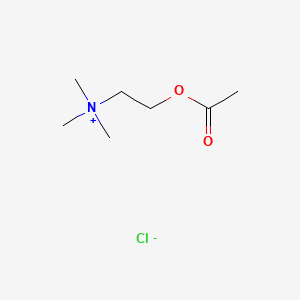

1. 2-(acetyloxy)-n,n,n-trimethylethanaminium
2. Acetilcolina Cusi
3. Acetylcholine
4. Acetylcholine Bromide
5. Acetylcholine Fluoride
6. Acetylcholine Hydroxide
7. Acetylcholine Iodide
8. Acetylcholine L Tartrate
9. Acetylcholine L-tartrate
10. Acetylcholine Perchlorate
11. Acetylcholine Picrate
12. Acetylcholine Picrate (1:1)
13. Acetylcholine Sulfate (1:1)
14. Bromide, Acetylcholine
15. Bromoacetylcholine
16. Chloroacetylcholine
17. Cusi, Acetilcolina
18. Fluoride, Acetylcholine
19. Hydroxide, Acetylcholine
20. Iodide, Acetylcholine
21. L-tartrate, Acetylcholine
22. Miochol
23. Perchlorate, Acetylcholine
1. 60-31-1
2. Miochol
3. Acecoline
4. Ach Chloride
5. Chloroacetylcholine
6. Arterocoline
7. Acecholin
8. Ovisot
9. 2-(acetyloxy)-n,n,n-trimethylethanaminium Chloride
10. Azetylcholinchlorid
11. Acetylcholine (chloride)
12. Choline Acetate (ester) Chloride
13. Ethanaminium, 2-(acetyloxy)-n,n,n-trimethyl-, Chloride
14. 2-acetoxyethyltrimethylammonium Chloride
15. Miochol-e
16. Acetylcholinium Chloride
17. O-acetylcholine Chloride
18. Choline, Chloride Acetate
19. Acetylcholine Hydrochloride
20. Acetylcholinechloride
21. Acetilcolina Cloruro [dcit]
22. (2-hydroxyethyl)trimethylammonium Chloride Acetate
23. Ach (chloride)
24. Acetylcholini Chloridum [inn-latin]
25. Chlorure D'acetylcholine [inn-french]
26. Cloruro De Acetilcolina [inn-spanish]
27. Tl 1505
28. Ai3-51676
29. Choline Acetate (ester), Chloride
30. Nsc-755845
31. 60-31-1 (chloride)
32. Ammonium, (2-hydroxyethyl)trimethyl-, Chloride, Acetate
33. Mls000028640
34. Chebi:2417
35. Af73293c2r
36. Acetylcholine Chloride For Injection
37. Smr000059182
38. (2-acetoxyethyl)trimethylammonium Chloride
39. 2-acetyloxy-n,n,n-trimethylethanaminium Chloride
40. Dsstox_cid_28904
41. Dsstox_rid_83172
42. Dsstox_gsid_48978
43. Acetylcholini Chloridum
44. Cas-60-31-1
45. Acetilcolina Cloruro
46. Cloruro De Acetilcolina
47. Chlorure D'acetylcholine
48. Ncgc00018123-05
49. Einecs 200-468-8
50. Vasil
51. Unii-af73293c2r
52. Ach;ach Chloride
53. Miochol (tn)
54. Mfcd00011698
55. Acetylcholine Chloride [ban:inn:jan]
56. 2-(trimethylamino)ethyl Acetate, Chloride
57. 2-acetyloxyethyl(trimethyl)azanium;chloride
58. Acetylcholine Chloride [usp:inn:ban:jan]
59. Opera_id_1682
60. Bmse000168
61. Chembl1184
62. Regid_for_cid_6060
63. Schembl40885
64. Spectrum1500104
65. Dtxsid5048978
66. Hms502c08
67. 2-(acetyloxy)-n,n,n-trimethylethanaminium Chloride (1:1)
68. Hms1920a09
69. Hms2091g09
70. Hms2230b10
71. Hms3259j11
72. Hms3371l03
73. Hms3651k19
74. Pharmakon1600-01500104
75. Acetylcholine Chloride [mi]
76. Bcp31537
77. Hy-b0282
78. Acetylcholine Chloride [inn]
79. Tox21_113435
80. Ccg-39125
81. Nsc755845
82. Acetylcholine Chloride [vandf]
83. Acetylcholine Chloride [mart.]
84. Akos005111031
85. Tox21_113435_1
86. Acetylcholine Chloride [usp-rs]
87. Acetylcholine Chloride [who-dd]
88. Nc00643
89. Nsc 755845
90. Acetylcholine Chloride (jp17/usp/inn)
91. Ncgc00018123-07
92. Ncgc00094577-01
93. Ncgc00094577-02
94. Ncgc00094577-03
95. Ds-11397
96. Acetylcholine Chloride [orange Book]
97. A0084
98. Acetylcholine Chloride [ep Monograph]
99. Acetylcholine Chloride [usp Impurity]
100. Ft-0621824
101. Ft-0661194
102. Sw220071-1
103. Acetylcholine Chloride [usp Monograph]
104. 2-acetoxy-n,n,n-trimethylethanaminium Chloride
105. C08201
106. D00999
107. D81773
108. 2-acetoxy-n,n,n-trimethylethan-1-aminium Chloride
109. Acetylcholine Chloride For Injection [jan]
110. Sr-01000003011
111. J-519537
112. Sr-01000003011-3
113. Q27105659
114. Ovisot; Tl 1505; Tl-1505; Tl-1505; Ach Chloride
115. Ethanaminium, 2-(acetyloxy)-n,n,n-trimethyl-, Chloride (1:1)
| Molecular Weight | 181.66 g/mol |
|---|---|
| Molecular Formula | C7H16ClNO2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 181.0869564 g/mol |
| Monoisotopic Mass | 181.0869564 g/mol |
| Topological Polar Surface Area | 26.3 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 115 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Miochol-e |
| PubMed Health | Acetylcholine (Into the eye) |
| Drug Classes | Direct Acting Miotic |
| Active Ingredient | Acetylcholine chloride |
| Dosage Form | For solution |
| Route | Ophthalmic |
| Strength | 20mg/vial |
| Market Status | Prescription |
| Company | Bausch And Lomb |
| 2 of 2 | |
|---|---|
| Drug Name | Miochol-e |
| PubMed Health | Acetylcholine (Into the eye) |
| Drug Classes | Direct Acting Miotic |
| Active Ingredient | Acetylcholine chloride |
| Dosage Form | For solution |
| Route | Ophthalmic |
| Strength | 20mg/vial |
| Market Status | Prescription |
| Company | Bausch And Lomb |
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
Cholinergic Agonists
Drugs that bind to and activate cholinergic receptors. (See all compounds classified as Cholinergic Agonists.)
