



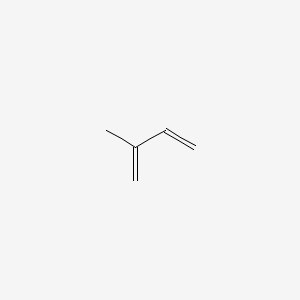

1. 2-methyl-1,3-butadiene
1. 2-methyl-1,3-butadiene
2. 78-79-5
3. 2-methylbuta-1,3-diene
4. Isopentadiene
5. 2-methylbutadiene
6. 1,3-butadiene, 2-methyl-
7. 2-methyldivinyl
8. Beta-methylbivinyl
9. Isopreno
10. Isoterpene
11. Isopren
12. 3-methyl-1,3-butadiene
13. .beta.-methylbivinyl
14. Ch2=c(ch3)ch=ch2
15. Rubber, Natural
16. Nsc 9237
17. Natural Rubber
18. 1,3-butadiene, 2-methyl-, Homopolymer
19. Chebi:35194
20. 0a62964ibu
21. Nsc-9237
22. 9006-04-6
23. Polyisoprene
24. Ccris 6253
25. Hsdb 620
26. Einecs 201-143-3
27. Un1218
28. Caoutchouc
29. Elastomers
30. Ebonite
31. Heveaplus
32. Impervia
33. Rubber
34. Latex Particles
35. Nafka
36. Natural Latex
37. Unii-0a62964ibu
38. India Rubber
39. Nafka Kristalgom
40. Latex Gum
41. Gum Nafkacrystal
42. Dynatex La
43. Dynatex Gtz
44. Thiokol Nvt
45. Nafka Crystal Gum
46. Latz Latex
47. Flexigum 40
48. 2-methyl-butadiene
49. Isoprene, Inhibited
50. Harub 5lv
51. Heveacrumb Smr 5l
52. 2-methyl-1
53. Lorival R 25
54. Hartex 102hr
55. Cartex 600
56. Fultite Fb 010k
57. Fultite Fb 520
58. Hartex 103
59. Fultite Fb 3001
60. Iotex C 60
61. Isoprene, >=99%
62. Kagetex Fa 2005
63. Isoprene [hsdb]
64. Isoprene [iarc]
65. E 218 (rubber)
66. Mitsuwa Rc Paper Cement
67. Isoprene [mi]
68. Defo 700
69. Dsstox_cid_761
70. Elastic Materials, Rubber
71. Unii-2lq0uuw8in
72. Lotol L 9241
73. Alkenes, Polymers, Rubber
74. 2lq0uuw8in
75. Be Be Tex 1223
76. Bmse000844
77. Defo 1000
78. Isna 5
79. Ec 201-143-3
80. 2-methyl-buta-1,3-diene
81. Ama 7
82. Csv 1
83. Dsstox_rid_75776
84. Dsstox_gsid_20761
85. Isoprene, Analytical Standard
86. Mar Dr 1135
87. 68877-32-7
88. 9003-31-0
89. Un 1218 (salt/mix)
90. Hydrocarbons, Polymers, Rubber
91. Drc 60
92. Chembl1566132
93. Dtxsid2020761
94. Wln: 1uy1&1u1
95. Hsdb 6772
96. Gln 200
97. Gnl 150
98. Isoprene (1 Mg/ml In Methanol)
99. Jlx 105
100. Jlx 113
101. Kdp 150
102. Nsc9237
103. Cv 50
104. Cv 60
105. Ir 25
106. Ir 68
107. Amy37001
108. Zinc1699876
109. Einecs 232-689-0
110. Tox21_200067
111. 5l-tp0203
112. Cs 700
113. Hc 106
114. Mfcd00008600
115. Akos000119971
116. Ccg-266006
117. Fb 3001
118. Cas-78-79-5
119. Ncgc00091078-01
120. Ncgc00091078-02
121. Ncgc00257621-01
122. Ft-0627457
123. I0160
124. Q271943
125. Isoprene, Inhibited [un1218] [flammable Liquid]
126. J-509898
127. Isoprene, 99%, Contains <1000 Ppm P-tert-butylcatechol As Inhibitor
128. 26796-44-1
| Molecular Weight | 68.12 g/mol |
|---|---|
| Molecular Formula | C5H8 |
| XLogP3 | 2.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 1 |
| Exact Mass | 68.062600255 g/mol |
| Monoisotopic Mass | 68.062600255 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 51.1 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Routine testing for latex allergy in patients with spina bifida is not recommended.
PMID:1990926 Slater JE, Mostello LA; Anesthesiology 74 (2): 391-2 (1991)
Studies on inhalation pharmacokinetics of isoprene were conducted in rats (Wistar) and mice (B6C3F1) to investigate possible species differences in metabolism of this compound. Pharmacokinetic analysis of isoprene inhaled by rats and mice revealed saturation kinetics of isoprene metabolism in both species. For rats and mice, linear pharmacokinetics apply at exposure concentrations below 300 ppm isoprene. Saturation of isoprene metabolism is practically complete at atmospheric concentrations of about 1000 ppm in rats and about 2000 ppm in mice. In the lower concentration range where first-order metabolism applies, metabolic clearance (related to the concentration in the atmosphere) of inhaled isoprene per kilogram body weight was 6200 mL/hr for rats and 12,000 mL/hr for mice. The estimated maximal metabolic elimination rates were 130 umole/hr/kg for rats and 400 umole/hr/kg for mice. This shows that the rate of isoprene metabolism in mice is about two or three times that in rats. When the untreated animals are kept in a closed all-glass exposure system, the exhalation of isoprene into the system can be measured. This shows that the isoprene endogenously produced by the animals is systemically available within the animal organism. From such experiments the endogenous production rate of isoprene was calculated to be 1.9 umole/hr/kg for rats and 0.4 umole/hr/kg for mice. /The/ data indicate that the endogenous production of isoprene should be accounted for when discussing a possible carcinogenic or mutagenic risk of this compound.
PMID:2401276 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1567771 Peter H et al; Environ Health Perspect 86: 89-92 (1990)
Male B6C3F1 mice were exposed to nominal concentrations of 20, 200, and 2000 ppm isoprene or (14)C-isoprene for up to 6 hr. For all exposures, steady-state levels of isoprene were reached rapidly (ie, within 15 to 30 min) after the onset of exposure. The mean (+/- SE) steady-state blood levels of isoprene (identified by headspace analysis) for the 20, 200, and 2000 ppm exposures were 24.8 +/- 3.3, 830 +/- 51, and 6800 +/- 400 ng isoprene/mL blood, respectively. At the two higher exposure concentrations, the increases in blood levels of isoprene were proportional to the increases in air concentrations of isoprene. There was approximately a 2.3-fold decrease in the retained (14)C/inhaled (14)C ratio with increasing exposure concentration. Depending on the exposure concentration, from 52% (20 ppm isoprene) to 73% (2000 ppm isoprene) of the metabolite-associated (nonisoprene) radioactivity was excreted in the urine over a 64-hr postexposure period. (14)C-O2 exhalation after the end of the 6-hr exposure was minimal (2%) at the 20 ppm exposure and increased up to 18% at the higher isoprene exposure concentrations. These data suggest that metabolism of isoprene in mice is nonlinear within the range of exposure concentrations used in this study. Hemoglobin adduct formation reached near-maximum between 200 and 2000 ppm isoprene exposure concentration, consistent with /the/ conclusion that pathways for metabolism of isoprene were saturated. Isoprene metabolites were present in blood after inhalation of isoprene at all concentrations studied. There were substantial differences in the toxicokinetics of inhaled isoprene in mice compared to rats. In mice, fractional retention of inhaled isoprene, which reflects, in part, metabolism of isoprene, was linearly related to exposure concentrations up to 200 ppm but decreased at 2000 ppm; in rats, fractional retention of inhaled isoprene decreased with increasing exposure concentration over a range of exposures from 8 to 1500 ppm.
PMID:2000636 Bond JA et al; Toxicol Appl Pharmacol 107 (3): 494-503 (1991)
The percent of the inhaled isoprene that was metabolized decreased with increasing exposure concn & vapor concn. About 75% of the total metabolites was excreted in urine. This was independent of inhaled isoprene concn. After exposure to 8200 ppm, a larger percent of the metabolites was excreted in feces than after exposure to lower concns. A mutagenic metabolite, isoprene diepoxide, was tentatively identified in all tissues examined. The relative amount of the metabolites present in blood was highest for low concns of inhaled isoprene & for shorter exposure durations. Body fat appeared to be a reservoir for both isoprene metabolites & isoprene itself.
PMID:3603560 Dahl AR et al; Toxicol Appl Pharmacol 89 (2): 237-48 (1987)
Isoprene is formed endogenously at the rate of 1.9 umol/kg per hour in both rats and mice.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V71 1019 (1999)
For more Absorption, Distribution and Excretion (Complete) data for Isoprene (7 total), please visit the HSDB record page.
Isoprene (IP) ... is metabolized in vivo to reactive epoxides, which might cause the tumors observed in IP exposed rodents. Detailed knowledge of the body and tissue burden of inhaled IP and its intermediate epoxides can be gained using a physiological toxicokinetic (PT) model. For this purpose, a PT-model was developed for IP in mouse, rat, and human. Experimentally determined partition coefficients were taken from the literature. Metabolic parameters were obtained from gas-uptake experiments. The measured data could be described by introducing hepatic and extrahepatic metabolism into the model. At exposure concentrations up to 50 ppm, the rate of metabolism at steady-state is 14 times faster in mice and about 8 times faster in rats than in humans (2.5 umol/hr/kg at 50 ppm IP in air). IP does accumulate only barely due to its fast metabolism and its low thermodynamic partition coefficient whole body:air. IP is produced endogenously. This production is negligible in rodents compared to that in humans (0.34 umol/hr/kg). About 90% of IP produced endogenously in humans is metabolized and 10% is exhaled unchanged. The blood concentration of IP in non-exposed humans is predicted to be 9.5 nmol/L. The area under the blood concentration-time curve (AUC) following exposure over 8 hr to 10 ppm IP is about 4 times higher than the AUC resulting from the unavoidable endogenous IP over 24 hr...
PMID:11397422 Csanady GA, Filser JG; Chem Biol Interact 135-136: 679-85 (2001)
...It is often speculated that the toxicological properties of isoprene must resemble those of butadiene. In fact, the acute toxicity of isoprene is very similar and also the biotransformation to mono- and diepoxides is qualitatively alike. There is however a difference; isoprene is asymmetric and therefore more metabolic enantiomers are possible. Pharmacokinetic studies have demonstrated species differences (as with butadiene) in the maximum metabolic elimination rate: in mice this was determined to be at least three times higher than in rats which implies a species sensitivity in isoprene metabolism in the mouse.
PMID:8901904 Taalman RD; Toxicology 113 (1-3): 242-6 (1996)
The metabolism of isoprene was investigated with microsomes derived from cell lines expressing human CYP1A1, CYP1A2, CYP2A6, CYP2B6, CYP2C9, CYP2D6, CYP2E1, or CYP3A4. The formation of epoxide metabolites was determined by gas chromatographic analysis. CYP2E1 showed the highest rates of formation of the isoprene monoepoxides 3,4-epoxy-3-methyl-1-butene (EPOX-I) and 3,4-epoxy-2-methyl-1-butene (EPOX-II), followed by CYP2B6. CYP2E1 was the only enzyme showing detectable formation of the diepoxide of isoprene, 2-methyl-1,2:3,4-diepoxybutane. Both isoprene monoepoxides were oxidized by CYP2E1 to the diepoxide at similar enzymatic rates. In order to determine the relative role of CYP2E1 in hepatic metabolism, isoprene as well as the two monoepoxides were also incubated with a series of ten human liver microsomal preparations in the presence of the epoxide hydrolase inhibitor cyclohexene oxide. The obtained activities were correlated with activities towards specific substrates for CYP1A2, CYP2A6, CYP2C9, CYP2D6, CYP2E1 and CYP3A. The results were supportive for those obtained with single human P450 enzymes. Isoprene (monoepoxide) metabolism sowed a significant correlation with CYP2E1 activity, determined as chlorzoxazone 6-hydroxylation. CYP2E1 is therefore the major enzyme involved in hepatic metabolism of isoprene and the isoprene monoepoxides in vitro. To investigae species differences with regard to the role of epoxide hydrolase in the metabolism of isoprene monoepoxides, the epoxidation of isoprene by human liver microsomes was compared to that of mouse and rate liver microsomes. The amounts of monoepoxides formed as a balance between epoxidation and hydrolysis, was measured in incubations with and without the epoxide hydrolase inhibitor cyclohexene oxide. Inhibition of epoxide hydrolase resulted in similar rates of monoepoxide formation in mouse, rat and man. Without inhibitor, however, the total amount of monoepoxides present at the end of the incubation period was twice as high for mouse liver microsomes than for rat and even 15 times as high as for human liver microsomes. Thus, differences in epoxide hydrolase activity between species may be of crucial importance for the toxicity of isoprene in the various species.
PMID:9021169 Bogaards JJ et al; Chem Biol Interact 102 (3): 169-82 (1996)
Comparative studies on the stereochemistry of the metabolism of isoprene in vitro have been carried out using liver microsomes from rats, mice, monkeys, dogs, rabbits and humans. Differences between strains and gender were also investigated. In the production of the isoprene monoepoxides, microsomes from the livers of the male Sprague-Dawley or Wistar rat showed an approximately 2:1 preference for the formation of (S)-2-(1-methylethenyl)oxirane compared with the (R)-enantiomer. No enantioselectivity was observed for mouse or rabbit. In contrast, liver microsomes from dog, monkey or male human preferentially formed (R)-2-(1-methylethenyl)oxirane. There was no enantioselectivity observed with microsomes from female human liver. The significant differences between species in the in vitro metabolism of isoprene indicate that stereochemical and mechanistic data should be taken into account when evaluating the results of animal studies designed to assess the carcinogenic risks to humans that may be associated with exposure to isoprene.
PMID:9413919 Small RD et al; Xenobiotica 27 (11): 1155-64 (1997)
For more Metabolism/Metabolites (Complete) data for Isoprene (13 total), please visit the HSDB record page.
The maximal metabolic elimination rates estimated from experiments in a closed inhalation system were 130 & 400 umol/kg/hr in male Wistar rats & B6C3F1 mice, respectively. The half-lives of isoprene were 6.8 min in rats & 4.4 min in mice.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V60 222 (1994)