



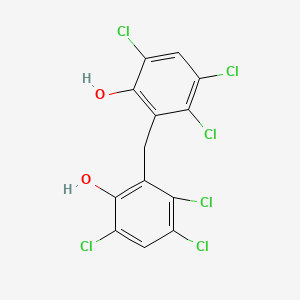

1. Hexachlorophane
1. 70-30-4
2. Hexachlorofen
3. Hexachlorophen
4. Nabac
5. Phisohex
6. Almederm
7. Gamophen
8. Septisol
9. Exofene
10. Fostril
11. Turgex
12. Germa-medica
13. Gamophene
14. Hexophene
15. Steraskin
16. Tersaseptic
17. Acigena
18. Dermadex
19. Distodin
20. Hexabalm
21. Hexafen
22. Phisodan
23. Septofen
24. Surofene
25. Hexide
26. Steral
27. Soy-dome
28. Surgi-cen
29. Surgi-cin
30. Hexachlorophane
31. Cotofilm
32. Hexascrub
33. Ritosept
34. Hexosan
35. Fomac
36. Fesia-sin
37. Phiso-scrub
38. Septi-soft
39. Ster-zac
40. Hexa-germ
41. Neosept V
42. G-eleven
43. Bilevon
44. Hexaclorofeno
45. Pre-op
46. Armohex
47. Hexachlorophenum
48. E-z Scrub
49. Compound G-11
50. 6,6'-methylenebis(2,4,5-trichlorophenol)
51. Nabac 25 Ec
52. Staphene O
53. Solu-heks
54. Esaclorofene
55. G-ii
56. 2,2'-methylenebis(3,4,6-trichlorophenol)
57. Scrubteam Surgical Spongebrush
58. At-17
59. Rcra Waste Number U132
60. 2,2'-methanediylbis(3,4,6-trichlorophenol)
61. At-7
62. Bis(2-hydroxy-3,5,6-trichlorophenyl)methane
63. Bis(3,5,6-trichloro-2-hydroxyphenyl)methane
64. Nci-c02653
65. G-11
66. 3,4,6-trichloro-2-[(2,3,5-trichloro-6-hydroxyphenyl)methyl]phenol
67. 2,2',3,3',5,5'-hexachloro-6,6'-dihydroxydiphenylmethane
68. 2,2'-dihydroxy-3,5,6,3',5',6'-hexachlorodiphenylmethane
69. 2,2'-dihydroxy-3,3',5,5',6,6'-hexachlorodiphenylmethane
70. Mls000028433
71. Smr000058356
72. Phenol, 2,2'-methylenebis(3,4,6-trichloro-
73. Phenol, 2,2'-methylenebis[3,4,6-trichloro-
74. Iww5fv6nk2
75. B 32
76. Mls002152906
77. Chebi:5693
78. 2,2'-methylenebis[3,4,6-trichlorophenol]
79. Bivelon
80. Trisophen
81. Hexaphene-lv
82. Hilo Flea Powder
83. Nsc-49115
84. Enditch Pet Shampoo
85. Ncgc00091195-04
86. Fascol
87. 6,6-methylenebis(2,4,5-trichlorophenol)
88. Esaclorofene [dcit]
89. Hexachlorofen [czech]
90. Hilo Cat Flea Powder
91. Dsstox_cid_690
92. Hexachlorophene [inn]
93. 2,4,6-trichlorophenol]
94. 2,5,6-trichlorophenol)
95. Pre-op Ii
96. Pedigree Dog Shampoo Bar
97. Dsstox_rid_75738
98. Dsstox_gsid_20690
99. Hexaclorofeno [inn-spanish]
100. Hexachlorophenum [inn-latin]
101. Caswell No. 566
102. Blockade Anti Bacterial Finish
103. At 7
104. B 32 (van)
105. Bis-2,5-trichlor-6-hydroxyfenylmethan
106. Cas-70-30-4
107. Methane,3,5-trichloro-6-hydroxyphenyl)
108. B & B Flea Kontroller For Dogs Only
109. Ccris 331
110. Bis(2-hydroxy-3,6-trichlorophenyl)methane
111. Bis(3,6-trichloro-2-hydroxyphenyl)methane
112. Phenol,2'-methylenebis[3,4,6-trichloro-
113. Phenol,2'-methylenebis[3,5,6-trichloro-
114. Wln: Qr Bg Dg Eg F1r Bq Cg Eg Fg
115. Hsdb 224
116. 2,3',5,5',6,6'-hexachlorodiphenylmethane
117. 2,5,6,3',5',6'-hexachlorodiphenylmethane
118. Sr-01000721924
119. Brevity Blue Liquid Sanitizing Scouring Cream
120. En-viron D Concentrated Phenolic Disinfectant
121. Einecs 200-733-8
122. Unii-iww5fv6nk2
123. Nsc 49115
124. Un2875
125. Hexachlorophene [un2875] [poison]
126. Phenol, 2,2'-methylenebis(3,4,6-trichloro)-
127. Rcra Waste No. U132
128. Brevity Blue Liquid Bacteriostatic Scouring Cream
129. 2,3,3',5,5'-hexachloro-6,6'-dihydroxydiphenylmethane
130. Epa Pesticide Chemical Code 044901
131. Bis-2,3,5-trichloro-6-hydroxyfenylmethan [czech]
132. Hexachlorophene (usp/inn)
133. Brn 2064407
134. Bis-2,3,5-trichloro-6-hydroxyfenylmethan
135. Ai3-02372
136. Hexachlorophenone
137. Thera-groom Pet Shampoo For Dogs For Veterinary Use Only
138. 2,2'-methylene Bis(3,4,6-trichlorophenol)
139. Hexachlorophene [usp:inn:ban]
140. Bis(3,5,6-trichloro-2-hydroxyphenyl)-methane
141. Methane, Bis(2,3,5-trichloro-6-hydroxyphenyl)
142. Kuc106447n
143. Methane, Bis(2,3,5-trichloro-6-hydroxyphenyl)-
144. Phisohex (tn)
145. Mfcd00002171
146. Ksc-19-051
147. Spectrum_000867
148. Bis-2,3,5-trichlor-6-hydroxyfenylmethan
149. Opera_id_504
150. Spectrum2_001105
151. Spectrum3_000450
152. Spectrum4_000573
153. Spectrum5_000792
154. M0219
155. Chembl496
156. Cid_3598
157. Hexachlorophene [mi]
158. Regid_for_cid_3598
159. Schembl15579
160. Bspbio_002100
161. Kbiogr_001006
162. Kbioss_001347
163. Mls001148404
164. Bidd:er0608
165. Bidd:gt0722
166. Divk1c_000630
167. Hexachlorophene [hsdb]
168. Hexachlorophene [iarc]
169. Hexachlorophene [inci]
170. Spectrum1500328
171. Spbio_001210
172. Hexachlorophene [vandf]
173. Amy389
174. Hexachlorophene [mart.]
175. Dtxsid6020690
176. Hexachlorophene [usp-rs]
177. Hexachlorophene [who-dd]
178. Bdbm31712
179. Gtpl11069
180. Hms501p12
181. Kbio1_000630
182. Kbio2_001347
183. Kbio2_003915
184. Kbio2_006483
185. Kbio3_001320
186. Nsc9887
187. Ninds_000630
188. 3,3',5,5',6,6'-hexachloro-2,2'-dihydroxydiphenylmethane
189. Hms1920d07
190. Hms2091j13
191. Hms3259c19
192. Hms3715p21
193. Kuc112427n
194. Pharmakon1600-01500328
195. Nsc-9887
196. Nsc49115
197. Zinc1530968
198. Tox21_111099
199. Tox21_201350
200. Tox21_302741
201. 3,4,6-trichloro-2-[(2,3,5-trichloro-6-hydroxy-phenyl)methyl]phenol
202. Ccg-39768
203. Hexachlorophene [orange Book]
204. Nsc757055
205. S4632
206. Stk377478
207. Akos005449243
208. Tox21_111099_1
209. Cs-3866
210. Db00756
211. Hexachlorophene [usp Monograph]
212. Nc00512
213. Nsc-757055
214. Un 2875
215. Idi1_000630
216. Ncgc00091195-01
217. Ncgc00091195-02
218. Ncgc00091195-03
219. Ncgc00091195-05
220. Ncgc00091195-06
221. Ncgc00091195-07
222. Ncgc00091195-08
223. Ncgc00256580-01
224. Ncgc00258902-01
225. As-10068
226. Bp-30177
227. H3p
228. Hy-12637
229. Inh 1 [pmid: 32284327]
230. Ksc-285-117-1
231. Sbi-0051403.p003
232. Ft-0626955
233. Bis(3,5,6-trichloro-2-hydroxy Phenyl)methane
234. Bis-(2-hydroxy-3,5,6-trichlorophenyl)methane
235. C08039
236. D00859
237. Mls-0072923.0001
238. Ab00052010_17
239. Bis[3,4,6-trichlorophenol], 2,2'-methylene-
240. Hexachlorophene, Pestanal(r), Analytical Standard
241. Q425362
242. Sr-01000721924-2
243. Sr-01000721924-5
244. Sr-01000721924-6
245. Brd-k99792991-001-02-6
246. Brd-k99792991-001-18-2
247. Hexachlorophene, United States Pharmacopeia (usp) Reference Standard
248. 139411-96-4
| Molecular Weight | 406.9 g/mol |
|---|---|
| Molecular Formula | C13H6Cl6O2 |
| XLogP3 | 7.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 405.846945 g/mol |
| Monoisotopic Mass | 403.849896 g/mol |
| Topological Polar Surface Area | 40.5 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 328 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Phisohex |
| PubMed Health | Hexachlorophene (Topical application route) |
| Drug Classes | Antibacterial Cleansing Agent |
| Active Ingredient | Hexachlorophene |
| Dosage Form | Emulsion |
| Route | Topical |
| Strength | 3% |
| Market Status | Prescription |
| Company | Sanofi Aventis Us |
| 2 of 6 | |
|---|---|
| Drug Name | Pre-op |
| Active Ingredient | Hexachlorophene |
| Dosage Form | Sponge |
| Route | Topical |
| Strength | 480mg |
| Market Status | Prescription |
| Company | Davis And Geck |
| 3 of 6 | |
|---|---|
| Drug Name | Pre-op ii |
| Active Ingredient | Hexachlorophene |
| Dosage Form | Sponge |
| Route | Topical |
| Strength | 480mg |
| Market Status | Prescription |
| Company | Davis And Geck |
| 4 of 6 | |
|---|---|
| Drug Name | Phisohex |
| PubMed Health | Hexachlorophene (Topical application route) |
| Drug Classes | Antibacterial Cleansing Agent |
| Active Ingredient | Hexachlorophene |
| Dosage Form | Emulsion |
| Route | Topical |
| Strength | 3% |
| Market Status | Prescription |
| Company | Sanofi Aventis Us |
| 5 of 6 | |
|---|---|
| Drug Name | Pre-op |
| Active Ingredient | Hexachlorophene |
| Dosage Form | Sponge |
| Route | Topical |
| Strength | 480mg |
| Market Status | Prescription |
| Company | Davis And Geck |
| 6 of 6 | |
|---|---|
| Drug Name | Pre-op ii |
| Active Ingredient | Hexachlorophene |
| Dosage Form | Sponge |
| Route | Topical |
| Strength | 480mg |
| Market Status | Prescription |
| Company | Davis And Geck |
Anti-Infective Agents, Local
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Hexachlorophene is used as a surgical hand scrub and a bacteriostatic skin cleanser. Topical preparations containing hexachlorophene have been used by physicians, dentists, food handlers, pediatric nurses, and other individuals who are in a position to spread contaminants from their hands. Efficacy of hexachlorophene as a topical antiseptic depends on residual amounts of the drug being adsorbed onto the skin. Hexachlorophene is most effective after repeated daily application and may be ineffective in reducing cutaneous flora if used in a single, brief application.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 3535
Hexachlorophene has been used topically to suppress staphylococcal infections in patients with acne vulgaris. However, hexachlorophene is not active against Propionibacterium acnes and is of no additional benefit in individuals using topical benzoyl peroxide or topical tetracycline, clindamycin, or erythromycin.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 3535
/SRP: Former use/ The use of hexachlorophene in the nursery has diminished sharply since discovery that daily bathing of neonates with 3% hexachlorophene emulsion could result in serious neurotoxicity. In hospitals in which ... /it/ is still used, the practice is to employ low concn (0.25%), which is less effective than 3%, or delay hexachlorophene bath until the 3rd day. Sometimes only the umbilical stump ... is bathed. The practice of subsequently rinsing off hexachlorophene residue with an alcohol or bathing the neonate with nonmedicated soap, in order to prevent absorption of hexachlorophene through the skin, defeats original purpose of use of antiseptic. ... Chlorhexidine ... is a more rational choice of antiseptic ... .
Gilman, A.G., L.S.Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 7th ed. New York: Macmillan Publishing Co., Inc., 1985., p. 969
For more Therapeutic Uses (Complete) data for HEXACHLOROPHENE (8 total), please visit the HSDB record page.
Food and Environmental Agents: Effect on Breast-Feeding: Hexachlorophene: None; possible contamination of milk from nipple washing. /from Table 7/
PMID:8265310 Committee on Drugs of the American Academy of Pediatrics: The Transfer of Drugs and Other Chemicals into Human Milk; Pediatrics 93 (1): 137-150 (1994)
Hexachlorophene should not be used routinely for prophylactic total body bathing. After use of hexachlorophene, the area, especially sensitive areas such as the scrotum and perineum, should be rinsed thoroughly. If hexachlorophene inadvertently gets into the eyes, the eyes should be flushed promptly and thoroughly with water.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 3535
Because of the possibility of rapid and extensive absorption of hexachlorophene, preparations containing the drug should not be used on burned or denuded skin or mucous membranes and should not be used as and/or with occlusive dressings, wet packs, lotions, vaginal packs, or tampons. In addition, hexachlorophene preparations should not be applied to generalized dermatologic conditions (e.g., lesions of ichthyosis congenita, dermatitis of Letterer-Siwe's syndrome).
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 3535
Hexachlorophene should not be used in individuals sensitive to the drug or any ingredient in the formulation. Because of the possibility of cross-sensitivity, hexachlorophene should not be used in individuals who have demonstrated primary light sensitivity to halogenated phenol derivatives.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 3535
For more Drug Warnings (Complete) data for HEXACHLOROPHENE (14 total), please visit the HSDB record page.
The lethal dose in adults after acute ingestion or repeated skin application is not well defined but is estimated to be 43 mg/kg or 1 to 10 g.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 1251
For use as a surgical scrub and a bacteriostatic skin cleanser. It may also be used to control an outbreak of gram-positive infection where other infection control procedures have been unsuccessful.
Hexachlorophene, a detergent cleanser, is an antibacterial sudsing emulsion for topical administration. It is a bacteriostatic cleansing agent. It cleanses the skin thoroughly and has bacteriostatic action against staphylococci and other gram-positive bacteria. Cumulative antibacterial action develops with repeated use. Cleansing with alcohol or soaps containing alcohol removes the antibacterial residue.
Anti-Infective Agents, Local
Substances used on humans and other animals that destroy harmful microorganisms or inhibit their activity. They are distinguished from DISINFECTANTS, which are used on inanimate objects. (See all compounds classified as Anti-Infective Agents, Local.)
D - Dermatologicals
D08 - Antiseptics and disinfectants
D08A - Antiseptics and disinfectants
D08AE - Phenol and derivatives
D08AE01 - Hexachlorophene
Absorption
Detectable blood levels of hexachlorophene following absorption through intact skin have been found in subjects who regularly scrubbed with hexachlorophene.
Hexachlorophene is absorbed systemically (percutaneously) following topical application to the skin. In one study, approximately 3% of a dose of hexachlorophene (in acetone) applied to the skin was absorbed systemically. Serum concentrations of hexachlorophene ranging from 0.009-4.35ug/mL have been reported in neonates bathed daily in hexachlorophene preparations for 1-56 days; highest concentrations occurred in low birthweight infants and infants with abraded or erythematous skin. In adults, 3-4 weeks of daily total body bathing with a 3% hexachlorophene preparation reportedly results in serum concentrations of the drug as high as 1.42 ug/mL. Hexachlorophene serum concentrations of 0.5 mcg/mL or higher have been reported following use of a 3% hexachlorophene preparation as a surgical scrub for hands and forearms 5 times daily for 10 days. In animals, characteristic changes in the CNS associated with hexachlorophene toxicity occur at serum drug concentrations of about 1 ug/mL or greater.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 3536
Following topical application to intact skin, hexachlorophene is adsorbed onto the outer layers of skin. Repeated daily application of hexachlorophene results in a residual of the drug being retained on the skin for several days. One study using radiolabeled hexachlorophene indicated that the drug accumulates on the skin during the first 3 or 4 days of repeated use, but the concentration on skin remains relatively constant thereafter. Residual hexachlorophene is retained on the skin for several days after discontinuance of the drug or may be removed by cleansing with non-hexachlorophene-containing soaps or detergents or ethanol or isopropyl alcohol.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 3536
Hexachlorophene is absorbed from the GI tract and from intact and denuded skin. Rapid absorption of hexachlorophene may occur following topical application to burned or inflamed skin.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 3536
Hexachlorophene was administered intraperitoneally to rats and rabbits. Excretion of this chemical was slow, most (48-83%) excreted unchanged in the feces.
Menzie, C.M. Metabolism of Pesticides-Update III. Special Scientific Report- Wildlife No. 232. Washington, DC: U.S.Department of the Interior, Fish and Wildlife Service, 1980., p. 323
For more Absorption, Distribution and Excretion (Complete) data for HEXACHLOROPHENE (14 total), please visit the HSDB record page.
Hexachlorophene is metabolized in the liver, although it apparently displays first-order (linear) kinetics.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 1251
The half life is 24 hours (range, 6 to 44 hours).
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 1251
In newborn infants exposed to soap containing hexachlorophene, its half-life ranged from 6-44 hr ... .
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V20 250 (1979)
The primary mechanism of action of hexachlorophene, based on studies with Bacillus megatherium, is to inhibit the membrane-bound part of the electron transport chain, respiratory D-lactate dehydrogenase. It induces leakage, causes protoplast lysis, and inhibits respiration.
Hexachlorophene ... /uncouples/ rat liver mitochondrial oxidative phosphorylation.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V20 248 (1979)
Brain succinate dehydrogenase (SDH) activity was inhibited by in vitro hexachlorophene (HCP) with a half inhibitory concentration (IC50) of 0.65 x 10(-3) M. The HCP exerted noncompetitive inhibition at 0.5 mM (
PMID:10487417 Lokanatha V et al; J Biochem Mol Toxicol 13 (6): 303-6 (1999)
Although the exact mechanism(s) of action has not been determined, at low concentrations, hexachlorophene appears to interrupt bacterial electron transport and to inhibit other membrane-bound enzymes. Higher concentrations rupture bacterial membranes.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 3536