



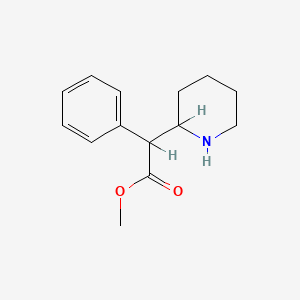

1. Centedrin
2. Concerta
3. Daytrana
4. Equasym
5. Hydrochloride, Methylphenidate
6. Metadate
7. Methylin
8. Methylphenidate Hydrochloride
9. Phenidylate
10. Ritalin
11. Ritalin Sr
12. Ritalin-sr
13. Ritaline
14. Tsentedrin
1. Methylphenidan
2. Phenidylate
3. Calocain
4. Plimasine
5. 113-45-1
6. Concerta
7. Methyl Phenidylacetate
8. Methyl Phenidate
9. Metilfenidato [italian]
10. Methylphenidatum
11. Metilfenidato [inn-spanish]
12. Methylphenidatum [inn-latin]
13. 4311/b Ciba
14. Methyl Phenidyl Acetate
15. Methyl Phenyl(piperidin-2-yl)acetate
16. Methylin
17. Methyl Alpha-phenyl-alpha-(2-piperidyl)acetate
18. Alpha-phenyl-2-piperidineacetic Acid Methyl Ester
19. Daytrana
20. Methylphenidate Hcl
21. Nci-c56280
22. 2-piperidineacetic Acid, Alpha-phenyl-, Methyl Ester
23. Methyl Alpha-phenyl-alpha-2-piperidinylacetate
24. Chembl796
25. 2-piperidineacetic Acid, .alpha.-phenyl-, Methyl Ester
26. Methyl 2-phenyl-2-(piperidin-2-yl)acetate
27. Methylfenidan
28. Chebi:84276
29. Metilfenidato
30. Methylphenidylacetate Hydrochloride
31. .alpha.-phenyl-2-piperidineacetic Acid Methyl Ester
32. D-methylphenidate Hcl
33. Cotempla Xr-odt
34. Daytrana (tn)
35. Threo-dl-methylphenidate
36. Methyl 2-phenyl-2-piperidin-2-ylacetate
37. Hsdb 3126
38. Methyl (2-phenyl-2-(2-piperidyl)acetate)
39. Prc-063
40. Einecs 204-028-6
41. C 4311
42. Methylphenidate Extended Release
43. Methylphenidate (usan/inn)
44. Rubifen
45. Alpha-phenyl-alpha-(2-piperidyl)acetic Acid Methyl Ester
46. Dea No. 1724
47. Methylphenidate [usan:inn:ban]
48. 40572-71-2
49. Ritalin (salt/mix)
50. Methylin (salt/mix)
51. Ritaline (salt/mix)
52. Centedein (salt/mix)
53. Centedrin (salt/mix)
54. Centedrine (salt/mix)
55. Schembl37178
56. Gtpl7236
57. Dtxsid5023299
58. Jornay Pm (a.k.a. Hld200)
59. Bcp18286
60. Hy-b1091
61. Bdbm50062912
62. Methyl Alpha-piperid-2-ylphenylacetate
63. Methyl Phenyl(2-piperidinyl)acetate #
64. Cs-4657
65. Db00422
66. Ncgc00248587-01
67. Ncgc00248587-03
68. Methyl .alpha.-phenyl-2-piperidineacetate
69. Sbi-0206868.p001
70. Methyl .alpha.-phenyl-2-piperidine-acetate
71. C07196
72. D04999
73. Phenyl-piperidin-2-yl-acetic Acid Methyl Ester
74. Ab01563134_01
75. 2-piperidineacetic Acid, ?-phenyl-, Methyl Ester
76. L001307
77. Q422112
78. Methyl .alpha.-phenyl-.alpha.-(2-piperidyl)acetate
79. Methyl .alpha.-phenyl-.alpha.-2-piperidinylacetate
80. (s,2s)--phenyl-2-piperidineacetic Acid Methyl Ester
81. Brd-a19585813-003-01-7
| Molecular Weight | 233.31 g/mol |
|---|---|
| Molecular Formula | C14H19NO2 |
| XLogP3 | 0.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 233.141578849 g/mol |
| Monoisotopic Mass | 233.141578849 g/mol |
| Topological Polar Surface Area | 38.3 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 249 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 20 | |
|---|---|
| Drug Name | Concerta |
| PubMed Health | Methylphenidate |
| Drug Classes | CNS Stimulant, Central Nervous System Agent |
| Drug Label | CONCERTA is a central nervous system (CNS) stimulant. CONCERTA is available in four tablet strengths. Each extended-release tablet for once-a-day oral administration contains 18, 27, 36, or 54 mg of methylphenidate HCl USP and is designed to have... |
| Active Ingredient | Methylphenidate hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 54mg; 18mg; 27mg; 36mg |
| Market Status | Prescription |
| Company | Janssen Pharms |
| 2 of 20 | |
|---|---|
| Drug Name | Daytrana |
| PubMed Health | Methylphenidate |
| Drug Classes | CNS Stimulant, Central Nervous System Agent |
| Drug Label | Daytrana is an adhesive-based matrix transdermal system (patch) that is applied to intact skin. The chemical name for methylphenidate is -phenyl-2-piperidineacetic acid methyl ester. It is a white to off-white powder and is soluble in alcohol, ethy... |
| Active Ingredient | Methylphenidate |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 30mg/9hr (3.3mg/hr); 15mg/9hr (1.6mg/hr); 10mg/9hr (1.1mg/hr); 20mg/9hr (2.2mg/hr) |
| Market Status | Prescription |
| Company | Noven Pharms |
| 3 of 20 | |
|---|---|
| Drug Name | Metadate cd |
| PubMed Health | Methylphenidate |
| Drug Classes | CNS Stimulant, Central Nervous System Agent |
| Drug Label | METADATE CD is a central nervous system (CNS) stimulant. The extended-release capsules comprise both immediate-release (IR) and extended-release (ER) beads such that 30% of the dose is provided by the IR component and 70% of the dose is provided by t... |
| Active Ingredient | Methylphenidate hydrochloride |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 30mg; 50mg; 60mg; 10mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Ucb |
| 4 of 20 | |
|---|---|
| Drug Name | Metadate er |
| PubMed Health | Methylphenidate (By mouth) |
| Drug Classes | CNS Stimulant, Central Nervous System Agent |
| Drug Label | METADATE ER Tablets (methylphenidate hydrochloride extended-release tablets, USP) are a mild central nervous system (CNS) stimulant. METADATE ER is available as 20 mg extended-release tablets for oral administration.Methylphenidate hydrochloride is m... |
| Active Ingredient | Methylphenidate hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 20mg |
| Market Status | Prescription |
| Company | Ucb |
| 5 of 20 | |
|---|---|
| Drug Name | Methylin |
| PubMed Health | Methylphenidate |
| Drug Classes | CNS Stimulant, Central Nervous System Agent |
| Drug Label | Methylphenidate hydrochloride is a mild central nervous system (CNS) stimulant, available for oral administration as tablets of 5 mg, 10 mg, and 20 mg and as extended-release tablets of 10 mg and 20 mg. Methylphenidate hydrochloride is methyl -phen... |
| Active Ingredient | Methylphenidate hydrochloride |
| Dosage Form | Tablet, chewable; Solution |
| Route | Oral |
| Strength | 2.5mg; 10mg/5ml; 5mg; 10mg; 5mg/5ml |
| Market Status | Prescription |
| Company | Mallinckrodt |
| 6 of 20 | |
|---|---|
| Drug Name | Methylin er |
| Drug Label | Methylphenidate hydrochloride is a mild central nervous system (CNS) stimulant, available for oral administration as tablets of 5 mg, 10 mg, and 20 mg and as extended-release tablets of 10 mg and 20 mg. Methylphenidate hydrochloride is methyl -phen... |
| Active Ingredient | Methylphenidate hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 10mg; 20mg |
| Market Status | Prescription |
| Company | Mallinckrodt |
| 7 of 20 | |
|---|---|
| Drug Name | Quillivant xr |
| Drug Label | QUILLIVANT XR is a powder that, after reconstitution with water, forms an extended-release oral suspension formulation of methylphenidate intended for once daily oral administration. QUILLIVANT XR contains approximately 20% immediate-release and 80%... |
| Active Ingredient | Methylphenidate hydrochloride |
| Dosage Form | For suspension, extended release |
| Route | Oral |
| Strength | 5mg/ml |
| Market Status | Prescription |
| Company | Nextwave Pharms |
| 8 of 20 | |
|---|---|
| Drug Name | Ritalin |
| Drug Label | Ritalin hydrochloride, methylphenidate hydrochloride USP, is a mild central nervous system (CNS) stimulant, available as tablets of 5, 10, and 20 mg for oral administration; Ritalin-SR is available as sustained-release tablets of 20 mg for oral admin... |
| Active Ingredient | Methylphenidate hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 5mg; 10mg; 20mg |
| Market Status | Prescription |
| Company | Novartis |
| 9 of 20 | |
|---|---|
| Drug Name | Ritalin la |
| Drug Label | Methylphenidate hydrochloride is a central nervous system (CNS) stimulant. Ritalin LA (methylphenidate hydrochloride) extended-release capsules is an extended-release formulation of methylphenidate with a bi-modal release profile. Ritalin LA uses t... |
| Active Ingredient | Methylphenidate hydrochloride |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 30mg; 10mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Novartis |
| 10 of 20 | |
|---|---|
| Drug Name | Ritalin-sr |
| Active Ingredient | Methylphenidate hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 20mg |
| Market Status | Prescription |
| Company | Novartis |
| 11 of 20 | |
|---|---|
| Drug Name | Concerta |
| PubMed Health | Methylphenidate |
| Drug Classes | CNS Stimulant, Central Nervous System Agent |
| Drug Label | CONCERTA is a central nervous system (CNS) stimulant. CONCERTA is available in four tablet strengths. Each extended-release tablet for once-a-day oral administration contains 18, 27, 36, or 54 mg of methylphenidate HCl USP and is designed to have... |
| Active Ingredient | Methylphenidate hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 54mg; 18mg; 27mg; 36mg |
| Market Status | Prescription |
| Company | Janssen Pharms |
| 12 of 20 | |
|---|---|
| Drug Name | Daytrana |
| PubMed Health | Methylphenidate |
| Drug Classes | CNS Stimulant, Central Nervous System Agent |
| Drug Label | Daytrana is an adhesive-based matrix transdermal system (patch) that is applied to intact skin. The chemical name for methylphenidate is -phenyl-2-piperidineacetic acid methyl ester. It is a white to off-white powder and is soluble in alcohol, ethy... |
| Active Ingredient | Methylphenidate |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 30mg/9hr (3.3mg/hr); 15mg/9hr (1.6mg/hr); 10mg/9hr (1.1mg/hr); 20mg/9hr (2.2mg/hr) |
| Market Status | Prescription |
| Company | Noven Pharms |
| 13 of 20 | |
|---|---|
| Drug Name | Metadate cd |
| PubMed Health | Methylphenidate |
| Drug Classes | CNS Stimulant, Central Nervous System Agent |
| Drug Label | METADATE CD is a central nervous system (CNS) stimulant. The extended-release capsules comprise both immediate-release (IR) and extended-release (ER) beads such that 30% of the dose is provided by the IR component and 70% of the dose is provided by t... |
| Active Ingredient | Methylphenidate hydrochloride |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 30mg; 50mg; 60mg; 10mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Ucb |
| 14 of 20 | |
|---|---|
| Drug Name | Metadate er |
| PubMed Health | Methylphenidate (By mouth) |
| Drug Classes | CNS Stimulant, Central Nervous System Agent |
| Drug Label | METADATE ER Tablets (methylphenidate hydrochloride extended-release tablets, USP) are a mild central nervous system (CNS) stimulant. METADATE ER is available as 20 mg extended-release tablets for oral administration.Methylphenidate hydrochloride is m... |
| Active Ingredient | Methylphenidate hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 20mg |
| Market Status | Prescription |
| Company | Ucb |
| 15 of 20 | |
|---|---|
| Drug Name | Methylin |
| PubMed Health | Methylphenidate |
| Drug Classes | CNS Stimulant, Central Nervous System Agent |
| Drug Label | Methylphenidate hydrochloride is a mild central nervous system (CNS) stimulant, available for oral administration as tablets of 5 mg, 10 mg, and 20 mg and as extended-release tablets of 10 mg and 20 mg. Methylphenidate hydrochloride is methyl -phen... |
| Active Ingredient | Methylphenidate hydrochloride |
| Dosage Form | Tablet, chewable; Solution |
| Route | Oral |
| Strength | 2.5mg; 10mg/5ml; 5mg; 10mg; 5mg/5ml |
| Market Status | Prescription |
| Company | Mallinckrodt |
| 16 of 20 | |
|---|---|
| Drug Name | Methylin er |
| Drug Label | Methylphenidate hydrochloride is a mild central nervous system (CNS) stimulant, available for oral administration as tablets of 5 mg, 10 mg, and 20 mg and as extended-release tablets of 10 mg and 20 mg. Methylphenidate hydrochloride is methyl -phen... |
| Active Ingredient | Methylphenidate hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 10mg; 20mg |
| Market Status | Prescription |
| Company | Mallinckrodt |
| 17 of 20 | |
|---|---|
| Drug Name | Quillivant xr |
| Drug Label | QUILLIVANT XR is a powder that, after reconstitution with water, forms an extended-release oral suspension formulation of methylphenidate intended for once daily oral administration. QUILLIVANT XR contains approximately 20% immediate-release and 80%... |
| Active Ingredient | Methylphenidate hydrochloride |
| Dosage Form | For suspension, extended release |
| Route | Oral |
| Strength | 5mg/ml |
| Market Status | Prescription |
| Company | Nextwave Pharms |
| 18 of 20 | |
|---|---|
| Drug Name | Ritalin |
| Drug Label | Ritalin hydrochloride, methylphenidate hydrochloride USP, is a mild central nervous system (CNS) stimulant, available as tablets of 5, 10, and 20 mg for oral administration; Ritalin-SR is available as sustained-release tablets of 20 mg for oral admin... |
| Active Ingredient | Methylphenidate hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 5mg; 10mg; 20mg |
| Market Status | Prescription |
| Company | Novartis |
| 19 of 20 | |
|---|---|
| Drug Name | Ritalin la |
| Drug Label | Methylphenidate hydrochloride is a central nervous system (CNS) stimulant. Ritalin LA (methylphenidate hydrochloride) extended-release capsules is an extended-release formulation of methylphenidate with a bi-modal release profile. Ritalin LA uses t... |
| Active Ingredient | Methylphenidate hydrochloride |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 30mg; 10mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Novartis |
| 20 of 20 | |
|---|---|
| Drug Name | Ritalin-sr |
| Active Ingredient | Methylphenidate hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 20mg |
| Market Status | Prescription |
| Company | Novartis |
Central Nervous System Stimulants; Dopamine Uptake Inhibitors
National Library of Medicine's Medical Subject Headings. Methylphenidate. Online file (MeSH, 2015). Available from, as of November 20, 2015: https://www.nlm.nih.gov/mesh/2015/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Methylphenidate is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of September 30, 2015: https://clinicaltrials.gov/search/intervention=methylphenidate
MEDICATION (VET): Methylphenidate may be useful for diagnosing and treating cataplexy/narcolepsy or hyperactivity in dogs.
Plumb D.C. Veterinary Drug Handbook. 8th ed. (pocket). Ames, IA: Wiley-Blackwell, 2015., p. 957
Treatment of cocaine abuse with agonists such as methylphenidate has been attempted, but with litle success except in patients with attention deficit disorders.
DHHS/NIDA; Research Monograph Series 88: Mechanisms of Cocaine Abuse and Toxicity p.125 (1988) DHHS Pub No. (ADM)89-1585
For more Therapeutic Uses (Complete) data for METHYLPHENIDATE (7 total), please visit the HSDB record page.
Methylphenidate should be used with caution in patients with hypertension. Blood pressure should be monitored at appropriate intervals in patients receiving the drug, especially those with hypertension.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2552
Hypersensitivity reactions including rash, macular rash, urticaria, fever, arthralgia, exfoliative dermatitis, erythema multiforme with histopathologic findings of necrotizing vasculitis, and thrombocytopenic purpura may occur in patients receiving methylphenidate. Stevens-Johnson syndrome has been reported rarely. Fixed drug eruption, angioedema, anaphylactic reaction, auricular swelling, bullous conditions, pruritus, eruptions, and exanthemas have been reported in patients receiving methylphenidate, although a definite causal relationship has not been established. Erythema occurs in a majority of patients receiving methylphenidate as the transdermal system but generally causes minimal or no discomfort.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2551
Abnormal liver function, ranging from serum aminotransferase (transaminase) elevations to hepatic coma, has been reported in patients receiving methylphenidate, although a definite causal relationship has not been established. Hepatotoxicity was associated with methylphenidate therapy in at least one patient.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2551
The most frequent adverse effects of methylphenidate appear to be dose related and include nervousness and insomnia.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2551
For more Drug Warnings (Complete) data for METHYLPHENIDATE (23 total), please visit the HSDB record page.
Methylphenidate is indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) in patients 6 years of age and older and for the treatment of narcolepsy.
Methylphenidate is a racemic mixture comprised of the d- and l-isomers. The d-isomer is more pharmacologically active than the l-isomer. Radioligand binding studies demonstrate that binding of methylphenidate in the brain is localized to dopamine-rich areas, in particular in the prefrontal cortex which has been demonstrated to play a prominent role in ADHD pathophysiology. In a number of animal models, methylphenidate enhances locomotor activity and induces stereotypic behaviours.
Central Nervous System Stimulants
A loosely defined group of drugs that tend to increase behavioral alertness, agitation, or excitation. They work by a variety of mechanisms, but usually not by direct excitation of neurons. The many drugs that have such actions as side effects to their main therapeutic use are not included here. (See all compounds classified as Central Nervous System Stimulants.)
Dopamine Uptake Inhibitors
Drugs that block the transport of DOPAMINE into axon terminals or into storage vesicles within terminals. Most of the ADRENERGIC UPTAKE INHIBITORS also inhibit dopamine uptake. (See all compounds classified as Dopamine Uptake Inhibitors.)
N06BA04
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N06 - Psychoanaleptics
N06B - Psychostimulants, agents used for adhd and nootropics
N06BA - Centrally acting sympathomimetics
N06BA04 - Methylphenidate
Absorption
Concerta: Methylphenidate is readily absorbed. Following oral administration of Concerta, plasma methylhphenidate concentrations reach an initial maximum at about 1 hour followed by gradual ascending concentrations over the next 5-9 hours. Mean times to reach peak plasma concentrations across all doses of Concerta occurred between 6-10 hours. Once daily dosing minimizes the fluctuations between peak and trough concentrations associated with multiple doses of immediate-release methylphenidate treatments. Depending on the doses provided, Cmax was found to range from 6.0-15.0ng/mL, Tmax ranged from 8.1-9.4h, and AUC ranged from 50.4-121.5 ngh/mL in children. When provided as Concerta, methylphenidate is released through the patented Osmotic Controlled-Release Oral Delivery (OROS) system where 22% of the dose is provided as an immediate release and 78% is provided through a gradual release. OROS is comprised of an osmotically active trilayer core surrounded by a semipermeable membrane with an immediate-release drug overcoat. Within an aqueous environment, such as the stomach, the drug overcoat, which consists of 22% of the dose, dissolves within one hour, providing an initial immediate-release formulation of methylphenidate. Water then permeates through the membrane into the tablet core where the osmotically active polymer excipients expand, allowing methylphenidate to release slowly through the orifice over a period of 6-7 hours. Concerta also provides a sustained 10-12 hour effect, allowing for once-daily dosing. Biphentin: Methylphenidate is rapidly and extensively absorbed following oral administration, with peak blood levels obtained in 1-3 hours. When provided as Biphentin, methylphenidate is released through a multi-layer release delivery system (MLRTM) where 40% of the dose is provided as an immediate release and 60% is provided through a gradual release. Biphentin was designed to be an alternative to separate doses of immediate-release (IR) methylphenidate by providing a biphasic concentration-time profile when given as a single dose. The MLRTM release system allows for a sustained effect for 10-12 hours, allowing for once-daily dosing that covers the major times that ADHD impairment might occur (such as school, homework periods, during the workday, etc). Methylphenidate (immediate release): Methylphenidate hydrochloride is rapidly and extensively absorbed from the tablets following oral administration; however, owing to extensive first-pass metabolism, bioavailability is low (approx. 30%) and large individual differences exist (11-52%). In one study, the administration of methylphenidate hydrochloride with food accelerated absorption but had no effect on the amount absorbed. Peak plasma concentrations of 10.8 and 7.8 ng/mL were observed, on average, 2 hours after administration of 0.30 mg/kg in children and adults, respectively. Peak plasma concentrations showed marked variability between subjects. Both the area under the concentration-time curve (AUC), and the peak plasma concentrations (Cmax) showed dose-proportionality.
Route of Elimination
After oral administration of an immediate release formulation of methylphenidate, 78%-97% of the dose is excreted in the urine and 1%-3% in the feces in the form of metabolites within 48-96 hours. Only small quantities (<1%) of unchanged methylphenidate appear in the urine. Most of the dose is excreted in the urine as ritalinic acid (60%-86%), the remainder being accounted for by minor metabolites.
Volume of Distribution
Concerta: Plasma methylphenidate concentrations in adults decline bi-exponentially following oral administration. Biphentin: The apparent distribution volume of methylphenidate in children is approximately 20 L/kg, with substantial variability (11 to 33 L/kg). Methylphenidate (immediate release): The apparent distribution volume of methylphenidate in children was approximately 20 L/kg, with substantial variability (11-33 L/kg). The volume of distribution after an intravenous dose (Vss) is 2.23 L/kg for the racemate in healthy adult volunteers.
Clearance
The apparent mean systemic clearance after an oral dose is 10.2 and 10.5 L/h/kg in children and adults, respectively for a 0.3 mg/kg dose, and 0.565 L/h/kg after an intravenous dose of the racemate in healthy adult volunteers.
Methylphenidate HCl Oral Solution is readily absorbed. Following oral administration of Methylphenidate HCl Oral Solution, peak plasma methylphenidate concentrations are achieved at 1 to 2 hours.
NIH; DailyMed. Current Medication Information for METHYLPHENIDATE HCL ORAL SOLUTION- methylphenidate hydrochloride solution (Revised: May 2015). Available from, as of November 24, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7b372d74-7ba7-437a-be8c-6e8335b78818
Methylphenidate is readily absorbed after oral administration and reaches peak concentrations in plasma in about 2 hr.
Brunton, L. Chabner, B, Knollman, B. Goodman and Gillman's The Pharmaceutical Basis of Therapeutics, Twelth Edition, McGraw Hill Medical, New York, NY. 2011, p. 299
Men received 0.15 or 0.3 mg/kg of methylphenidate orally, and methylphenidate and ritalinic acid, a metabolite, were analyzed in plasma samples obtained at various times after treatment. Maximal methylphenidate concentrations in plasma were found to occur 2.2 hours after administration of either dose. The mean maximal concentration in plasma for methylphenidate was 3.5 ng/mL after 0.15 mg/kg, and 7.8 ng/mL after 0.3 mg/kg. Methylphenidate clearances were high (10.1 L/hour/kg) and variable (range: 3.6-23.2) for the 0.3 mg/kg dose. Pharmacokinetic parameters for children receiving 0.3 mg/kg were essentially the same as for the adults. Ritalinic acid plasma levels were 50-100 times greater than methylphenidate levels in normal adults. The clearance of ritalinic acid is less than that of methylphenidate. When admin to the rat, the absolute bioavailability of methylphenidate was found to be 0.19 in the rat and 0.22 in the monkey, suggesting substantial presystemic elimination of methylphenidate.
PMID:6410043 WARGIN W ET AL; J PHARMACOL EXP THER 226 (2): 382-6 (1983)
Neuroanatomical distribution of (14)C-labeled methylphenidate was examined in rabbit brain following intracerebroventricular administration at 15, 60, and 180 minutes after the injection. The highest levels were observed at the 1st sampling time (15 minutes) in medulla and cervical spinal cord. The pons, caudate, tegmentum, and hypothalamus also showed significant uptake of (14)C-methylphenidate.
SHAH NS ET AL; PROG NEURO-PSYCHOPHARMACOL BIOL PSYCHIATRY 7 (1): 101-6 (1983)
For more Absorption, Distribution and Excretion (Complete) data for METHYLPHENIDATE (7 total), please visit the HSDB record page.
Methylphenidate is hepatically metabolized. More specifically, it is rapidly and extensively metabolized by carboxylesterase CES1A1. Via this enzyme, methylphenidate undergoes de-esterification to ritalinic acid (a-phenyl-2-piperidine acetic acid, PPAA), which has little to no pharmacologic activity.
In humans, methylphenidate is metabolized primarily via deesterification to alpha-phenylpiperidine acetic acid (PPA, ritalinic acid). The metabolite has little or no pharmacologic activity.
NIH; DailyMed. Current Medication Information for METHYLPHENIDATE HCL ORAL SOLUTION- methylphenidate hydrochloride solution (Revised: May 2015). Available from, as of November 24, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7b372d74-7ba7-437a-be8c-6e8335b78818
Concerta: The half-life of methylphenidate in adults following oral administration of Concerta was approximately 3.5 h. Biphentin: Methylphenidate is eliminated from plasma with a mean half-life of 2.4 hours in children and 2.1 hours in adults. Methylphenidate (immediate release): Methylphenidate is eliminated from the plasma with a mean half-life of 2.4 hours in children and 2.1 hours in adults.
Methylphenidate ... is a racemate; /in plasma/ its more potent (+) enantimoer has a t(1/2) of approximately 6 hr, and the less potent (-) enantiomer has a t(1/2) of approximately 4 hr. Concentrations in the brain exceed those in plasma.
Brunton, L. Chabner, B, Knollman, B. Goodman and Gillman's The Pharmaceutical Basis of Therapeutics, Twelth Edition, McGraw Hill Medical, New York, NY. 2011, p. 299
The mean terminal half-life (t1/2) of methylphenidate following administration of 20 mg Methylphenidate HCl Oral Solution (t1/2 = 2.7 hours) is comparable to the mean terminal t1/2 following administration of Ritalin (methylphenidate hydrochloride immediate-release tablets) (t1/2 = 2.8h) in healthy adult volunteers.
NIH; DailyMed. Current Medication Information for METHYLPHENIDATE HCL ORAL SOLUTION- methylphenidate hydrochloride solution (Revised: May 2015). Available from, as of November 24, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7b372d74-7ba7-437a-be8c-6e8335b78818
While its exact mechanism is unclear, methylphenidate (MPH) has been shown to act as a norepinephrine and dopamine reuptake inhibitor (NDRI), thereby increasing the presence of these neurotransmitters in the extraneuronal space and prolonging their action. There is a dose-related effect of psychostimulants on receptor stimulation, where higher doses are shown to increase norepinephrine (NE) and dopamine (DA) efflux throughout the brain which can result in impaired cognition and locomotor-activating effects. In contrast, low doses are found to selectively activate NE and DE neurotransmission within the prefrontal cortex which is an area of the brain thought to play a prominent role in ADHD pathophysiology, thereby improving clinical efficacy and preventing side effects. The lower doses used to treat ADHD are not associated with the locomotor-activating effects associated with higher doses and instead reduce movement, impulsivity, and increase cognitive function including sustained attention and working memory. Methylphenidate's beneficial effects in sustaining attention have also been shown to be mediated by alpha-1 adrenergic receptor activity. Clinical findings have shown that children with ADHD have an abnormality in the dopamine transporter gene (DAT1), the D4 receptor gene (DRD-4), and/or the D2 receptor gene that may be at least partly overcome by the dopaminergic effects of methylphenidate, suggesting a possible mode of action.
Methylphenidate is thought to block the reuptake of norepinephrine and dopamine into the presynaptic neuron and increase the release of these monoamines into the extraneuronal space.
NIH; DailyMed. Current Medication Information for METHYLPHENIDATE HCL ORAL SOLUTION- methylphenidate hydrochloride solution (Revised: May 2015). Available from, as of November 24, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7b372d74-7ba7-437a-be8c-6e8335b78818
Although the primary mechanism is largely unknown, the effects of methylphenidate appear to be mediated by blockage of the reuptake mechanism of dopaminergic neurons. In children with attention deficit disorder, methylphenidate decreases motor restlessness and enhances the ability to pay attention. In narcolepsy, methylphenidate appears to act at the cerebral cortex and subcortical structures, including the thalamus, to produce CNS stimulation, resulting in increaed motor activity, increased mental alertness, diminished sense of fatigue, brighter spirits, and mild euphoria.
USP Convention. USPDI - Drug Information for the Health Care Professional. 17th ed. Volume I. Rockville, MD: Convention, Inc., 1997. (Plus Updates)., p. 2010
Mode of action: Appears to exert most or all of its effect in the CNS by causing release of biogenic amines, especially norepinephrine and dopamine, from storage sites in nerve terminals. It may also slow down catecholamine metabolism by inhibiting monoamine oxidase.
International Programme on Chemical Safety; Poisons Information Monograph: Methylphenidate Hydrochloride (PIM 344) (1998) Available from, as of October 24, 2005: https://www.inchem.org/pages/pims.html