



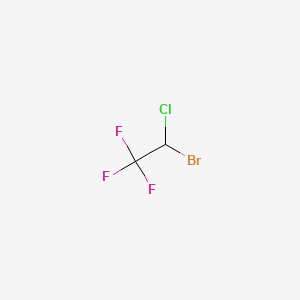

1. 1,1,1-trifluoro-2-chloro-2-bromoethane
2. Fluothane
3. Ftorotan
4. Narcotan
1. 151-67-7
2. 2-bromo-2-chloro-1,1,1-trifluoroethane
3. Fluothane
4. Narcotane
5. Rhodialothan
6. Narcotan
7. Anestan
8. Bromochlorotrifluoroethane
9. Fluorotane
10. Ftuorotan
11. Phthorothanum
12. Ftorotan
13. Halothan
14. Narkotan
15. Fluktan
16. Halotan
17. Halsan
18. Halan
19. Freon 123b1
20. Chalothane
21. Halotano
22. Halothanum
23. Alotano
24. Ftorotan [russian]
25. Alotano [dcit]
26. Bromchlortrifluoraethanum
27. Ethane, 2-bromo-2-chloro-1,1,1-trifluoro-
28. 1,1,1-trifluoro-2-chloro-2-bromoethane
29. 1-bromo-1-chloro-2,2,2-trifluoroethane
30. Halotano [inn-spanish]
31. Halothanum [inn-latin]
32. 1,1,1-trifluoro-2-bromo-2-chloroethane
33. 2,2,2-trifluoro-1-chloro-1-bromoethane
34. Narcotann Ne-spofa
35. Narcotann Ne-spofa [russian]
36. Cf3chclbr
37. Ethane, Bromochlorotrifluoro-
38. Nsc 143490
39. Chebi:5615
40. Ethane, 1-bromo-1-chloro-2,2,2-trifluoro-
41. Chembl931
42. Nsc-143490
43. Uqt9g45d1p
44. Ncgc00090868-01
45. Dsstox_cid_5371
46. Dsstox_rid_77767
47. Dsstox_gsid_25371
48. Fluothane (tn)
49. Cas-151-67-7
50. 2-brom-2-chlor-1,1,1-trifluorethan
51. Ccris 6244
52. Hsdb 6753
53. Halothane [anaesthetics, Volatile]
54. Einecs 205-796-5
55. Mfcd00009602
56. Unii-uqt9g45d1p
57. Brn 1736947
58. Halothane [usp:inn:ban:jan]
59. (+-)-2-bromo-2-chloro-1,1,1-trifluoroethane
60. Wln: Gyexfff
61. Halothane [inn]
62. Halothane [jan]
63. Halothane [mi]
64. Ethane,1,1-trifluoro-
65. Halon 2311
66. Halothane [hsdb]
67. Halothane [vandf]
68. Epitope Id:150925
69. Halothane [mart.]
70. (.+/-.))-halothane
71. Halothane [who-dd]
72. Halothane [who-ip]
73. Schembl25588
74. Halothane (jp17/usp/inn)
75. Halothane [green Book]
76. Gtpl2401
77. Halothane [ep Impurity]
78. Halothane [orange Book]
79. Dtxsid4025371
80. Ethane, 2-bromo-2-chloro-1,1,1-trifluoro-, (+-)-
81. Halothane [ep Monograph]
82. Bcqzxomgpxttic-uhfffaoysa-
83. Halothane [usp Monograph]
84. Halothanum [who-ip Latin]
85. Hms2094k17
86. Hms3885h08
87. Pharmakon1600-01505434
88. Hy-b1010
89. Tox21_111033
90. Tox21_200850
91. Bdbm50112212
92. Nsc143490
93. Nsc760138
94. S4570
95. Akos006227959
96. Tox21_111033_1
97. 1,1-trifluoro-2-bromo-2-chloroethane
98. 1,1-trifluoro-2-chloro-2-bromoethane
99. 2,2-trifluoro-1-chloro-1-bromoethane
100. 2-bromo-2-chloro-1,1-trifluoroethane
101. Ccg-213438
102. Cs-4523
103. Db01159
104. Nsc-760138
105. 1-bromo-1-chloro-2,2,2-trifluorethane
106. Ncgc00090868-02
107. Ncgc00090868-03
108. Ncgc00090868-05
109. Ncgc00090868-06
110. Ncgc00258404-01
111. Sbi-0206928.p001
112. Db-043111
113. Ft-0626853
114. C07515
115. D00542
116. H11280
117. Q32921
118. 1-bromo-1-chloro-2,2,2-trifluoroethane, 99%
119. Ab01563314_01
120. Sr-05000001969
121. 2-bromo-2-chloro-1,1,1-trifluoroethane, >=99%
122. J-008834
123. Sr-05000001969-1
124. (+/-)-2-bromo-2-chloro-1,1,1-trifluoroethane
125. 2-bromo-2-chloro-1,1,1-trifluoroethane, >=99.0% (gc)
126. Ethane, 2-bromo-2-chloro-1,1,1-trifluoro-,(+/-)-
127. Halothane, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 197.38 g/mol |
|---|---|
| Molecular Formula | C2HBrClF3 |
| XLogP3 | 2.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 195.89022 g/mol |
| Monoisotopic Mass | 195.89022 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 60.4 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anesthetics, Inhalation
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Anesthesia, general- ... halothane ... /is/ indicated for the induction and maintenance of general anesthesia. However, inhalation anesthetic agents are rarely used alone; other medications are frequently administered to induce or supplement anesthesia.. /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005.
For cesarean section: ... halothane ... /is/ indicated in low concentrations to supplement other general anesthetics during delivery by cesarean section./NOT included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005.
MEDICATION (VET): Halothane is a potent anesthetic and capable of maintaining anesthesia effectively in large animals. The level and depth of anesthesia can be more rapidly changed than as noted with methoxyflurane.
Booth, N.H., L.E. McDonald (eds.). Veterinary Pharmacology and Therapeutics. 5th ed. Ames, Iowa: Iowa State University Press, 1982., p. 187
Halothane diminishes sympathetic activity, augments vagal tone, depresses the contractility of the heart, and induces venodilation. Cardiac output, arterial pressure, and pulse rate are reduced, usually in proportion to the depth of anesthesia. Severe hypotension and circulatory failure may occur with overdosage. Supraventricular arrhythmias or nodal rhythm may be observed during induction or deep anesthesia. Small doses of epinephrine (1 to 1.5 ug/kg) may be administered subcutaneously or submucosally with halothane when adequate ventilation is assured. Exceeding this dose is potentially hazardous, however, since this anesthetic sensitizes the heart to catecholamines. The administration of lidocaine during epinephrine use decreases the risk of arrhythmias.
American Medical Association. AMA Drug Evaluations Annual 1991. Chicago, IL: American Medical Association, 1991., p. 162
Halothane should not be given to patients who developed jaundice or acute liver damage after previous exposure to this drug unless other obvious causes for the hepatic damage have been demonstrated.
American Medical Association. AMA Drug Evaluations Annual 1991. Chicago, IL: American Medical Association, 1991., p. 162
CONTRAINDICATIONS. Because of a probable adverse interaction, halothane should not be used in animals known to have recently received aminoglycoside antibiotics. Animals receiving phenobarbital or other potent enzyme-inducing drugs should not be subjected to halothane anesthesia because of potential injury to the liver (halothane hepatitis). Animals afflicted with cardiopathies or chronic congestive heart failure should not receive halothane. Until more information is available on behavioral effects of halothane, veterinary anesthesiologists probably should avoid its use in pregnant animals during the first and second trimesters.
Booth, N.H., L.E. McDonald (eds.). Veterinary Pharmacology and Therapeutics. 5th ed. Ames, Iowa: Iowa State University Press, 1982., p. 188
Halothane reduces muscle tone in the pregnant uterus and generally its use is not recommended in obsterics because of the increased risk of postpartum hemorrhage. Halothane should not be used for patients with cardiac arrhythmias.
Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 742
For more Drug Warnings (Complete) data for 2-BROMO-2-CHLORO-1,1,1-TRIFLUOROETHANE (12 total), please visit the HSDB record page.
For the induction and maintenance of general anesthesia
Halothane is a general inhalation anesthetic used for induction and maintenance of general anesthesia. It reduces the blood pressure and frequently decreases the pulse rate and depresses respiration. It induces muscle relaxation and reduces pains sensitivity by altering tissue excitability. It does so by decreasing the extent of gap junction mediated cell-cell coupling and altering the activity of the channels that underlie the action potential.
Anesthetics, Inhalation
Gases or volatile liquids that vary in the rate at which they induce anesthesia; potency; the degree of circulation, respiratory, or neuromuscular depression they produce; and analgesic effects. Inhalation anesthetics have advantages over intravenous agents in that the depth of anesthesia can be changed rapidly by altering the inhaled concentration. Because of their rapid elimination, any postoperative respiratory depression is of relatively short duration. (From AMA Drug Evaluations Annual, 1994, p173) (See all compounds classified as Anesthetics, Inhalation.)
N - Nervous system
N01 - Anesthetics
N01A - Anesthetics, general
N01AB - Halogenated hydrocarbons
N01AB01 - Halothane
Most halothane is excreted by the lung unchanged. At least 12% of an absorbed dose is metabolized to chlorine, bromine, and trifluoroacetic acid, with toxic intermediates suspected of causing or contributing to hepatoxicity. Halothane is stored in fatty tissue and has been detected in the expired air of obese patients up to 2 weeks after exposure.
Ellenhorn, M.J. and D.G. Barceloux. Medical Toxicology - Diagnosis and Treatment of Human Poisoning. New York, NY: Elsevier Science Publishing Co., Inc. 1988., p. 977
Urinary oxalate crystals were detected in 6 of 14 patients given halothane.
Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 741
It is not known if halothane is distributed into breast milk.
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005.
60 to 80% Excreted unchanged by exhalation.
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005.
Inhalation anesthetics cross placenta.
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005.
Halothane is metabolized in the liver, primarily by CYP2E1, and to a lesser extent by CYP3A4 and CYP2A6.
Anywhere from 10 to 30 percent of inhaled halothane is metabolized, and metabolites may be detected in the urine for a period of several days after inhaling halothane. Various intermediate metabolites have been isolated; however, trifluoroacetic acid is the principal end-product isolated from the urine.
Haddad, L.M., Clinical Management of Poisoning and Drug Overdose. 2nd ed. Philadelphia, PA: W.B. Saunders Co., 1990., p. 1171
Halothane biotransformation by cytochrome p 450 produces reactive intermediates along both oxidative (acyl chloride) and reductive (free radical) pathways that ultimately generate the metabolites trifluoroacetic acid and flouride, respectively. Inhibiting oxidative metabolism with deuterated halothane reduces resultant injury in our guinea pig model of acute halothane hepatoxicity. To elucidate whether covalent binding of reactive intermediates to proteins (oxidative pathway) or lipids (reductive pathway) is a mechanism of necrosis, male outbred Hartley guinea pigs (600-725 g), N = 8, were exposed to either 1% (v/v) halothane or deuterated halothane at either 40% or 10% oxygen for 4 hr. One-half of the animals were killed immediately after exposure for binding studies; the remainder at 96 hr post exposure for evaluation of hepatotoxicity. Covalent binding of halothane intermediates to liver protein or lipid was determined by measuring the fluoride content of the bound moieties. The use of deuterated halothane and/or 10% oxygen during exposure led to 63-88% reductions (p< 0.01) in plasma trifluoroacetic acid concn (halothane-40% oxygen = 546; 73 mM, N = 8) which were accompanied by 33-60% decreases (p< 0.01) in binding to liver proteins (halothane-40% oxygen = 1.36; 0.26 nmoles bound fluoride/mg protein, N = 4), 78-84% decreases (p< 0.05) in 48 hr plasma ALT levels (halothane- 40% oxygen = 308; 219, control = 23 + 3, N = 4) and a total amelioration of centilobular necrosis.
PMID:2069053 Lind RC, Gandolfi AJ; Adv Exp Med Biol 283: 763-6 (1991)
Free radicals were detected from the in vitro metabolism of halothane (rat liver microsomes) by the PBN spin trapping method. The detected radical species include the 1-chloro-2,2,2-trifluoro-1-ethyl radical (I), as determined by mass spectral analysis, and lipid type radicals assigned by high resolution ESR spectroscopy with the use of d14-deuterated PBN. The lipid derived radicals are a carbon centered radical with the partially assigned structure CH2R and an oxygen centered radical of the OR' type. From the mass spectral analysis of the spin adduct mixture there is also evidence for a halocarbon double adduct of PBN of the type I-PBN-I.
PMID:2167272 Janzen EG et al; Free Radic Res Commun 9 (3-6): 343-51 (1990)
An analogue of HCFC-123, the common inhalation anesthetic halothane (2-bromo-2-chloro-1,1,1-trifluoroethane), is metabolized by hepatic CYP2E1 to trifluoroacetyl chloride, causing trifluoroacetylation of liver proteins. These include cytochrome P450 itself and other enzymes, many of which have been identified as residing in the lumen of the endoplasmic reticulum and involved in the maturation of newly synthesized proteins. Both halothane and HCFC-123 induce peroxisome proliferation and increased beta-oxidation in rat liver cells. They are also highly effective in inducing excess uncoupled cytochrome P450 activity in rabbit liver microsomes, thus increasing hepatic oxygen consumption and facilitating the oxidation of other cytochrome P450 substrates.
International Programme on Chemical Safety's Concise International Chemical Assessment Documents. Number 23: 2,2-Dichloro-1,1,1-trifluoroethane (HCFC-123) (2000). Available from, as of October 13, 2005: https://www.inchem.org/pages/cicads.html
For more Metabolism/Metabolites (Complete) data for 2-BROMO-2-CHLORO-1,1,1-TRIFLUOROETHANE (7 total), please visit the HSDB record page.
Halothane has known human metabolites that include 2-chloro-1,1-difluoroethene, Trifluoroacetyl chloride, and chlorotrifluoroethane.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Halothane causes general anaethesia due to its actions on multiple ion channels, which ultimately depresses nerve conduction, breathing, cardiac contractility. Its immobilizing effects have been attributed to its binding to potassium channels in cholinergic neurons. Halothane's effect are also likely due to binding to NMDA and calcium channels, causing hyperpolarization.
The precise mechanism by which inhalation anesthetics produce loss of perception of sensations and unconsciousness is not known. Inhaled anesthetics act at many areas of the CNS. The Meyer-Overton theory suggests that the site of action of inhaled anesthetics may be the lipid matrix of neuronal membranes or other lipophilic sites. Anesthetics may cause changes in membrane thickness, which in turn effects the gating properties of ion channels in neurons. Interference with the hydrophobic portion of neuronal ion channel membrane proteins may be an important mechanism.
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005.
In vitro muscle contracture tests for malignant hyperthermia screening are routinely performed using standardized protocols. In the present study online monitoring of halothane concn in the gas phase was demonstrated to be an improved test standard. The kinetics of halothane concn and their effect on in vitro muscle contracture tests were evaluated in two test baths, I and II, which contained 3 and 18 ml Krebs-Ringer solution, respectively. The equilibration kinetics for halothane was significantly faster in bath I (half-life = 8.2 sec) compared with bath II (half-life = 25.6 sec). Twenty one pairs of muscle bundles from 21 potentially malignant hyperthermia susceptible patients were investigated, each test bath receiving one bundle from each pair. The variance of muscle contractures was significantly increased in test bath I compared with test bath II. However, there was no influence on malignant hyperthermia diagnosis, suggesting that, within the ranges of half-life = 8.2 sec-25.6 sec, the test bath volumes need not be standardized.
PMID:1549929 Urwyler A et al; Acta Anaesthesiol Scand 36 (2): 115-8 (1992)
Volatile anesthetics inhibit phagocytic cell function, yet little is known about their effects on target tissues or on the target tissue response to stimulated phagocytes. Experiments were performed to determine how exposure to halothane and isoflurane changes rat pulmonary artery endothelial cell viability in response to the toxic oxygen metabolites produced by stimulated phagocytic cells. Rat pulmonary arterial endothelial cells were grown in monolayer culture. The monolayers were treated with phorbol myristate acetate stimulated human neutrophils at an effector-to-target ratio of 20:1 after equilibration with 0.4% or 1.7% halothane or 0.7% or 2.8% isoflurane. As measured by percent specific release of incorporated (51)Cr label (mean + or - standard error), cytotoxicity in the presence of 1.7% halothane (75.3 + or - 3.4%) was significantly greater (p< 0.02) than cytotoxicity in 5% carbon dioxide in air (44.7 + or - 3.3%) and in 0.4% halothane (57.3 + or - 4.7%). Also, cytotoxicity in 1.7% halothane was significantly greater than in 0.4% halothane (p< 0.02). It was found that rat pulmonary arterial endothelial cells incubated in isoflurane exhibited significantly greater release of (51)Cr than cells incubated in the MAC equivalent concn of halothane: 78.2 + or - 2.6% in 0.7% isoflurane (p= 0.0004) and 83.8 + or - 1% in 2.8% isoflurane (p= 0.005). Because early neutrophil cytotoxicity has been found to be mediated primarily by hydroxyl radical and hydrogen peroxide, hydrogen peroxide production by similar numbers of phorbol myristate acetate stimulated neutrophils under similar exposure conditions was measured. In carrier gas, phorbol myristate acetate stimulated neutrophils produced 20.5 + or - 1.3 nmole hydrogen peroxide/1X10+6 cells/hr. At the higher concn of halothane, hydrogen peroxide production actually was inhibited in comparison with carrier gas (15.4 + or - 1.4 nmole hydrogen peroxide/1X10+6 cells/hr in 1.7% halothane and 16.8 + or - 0.8 in 2.8% halothane), but the degree of inhibition did not reach statistical significance. In isoflurane, however, hydrogen peroxide production was not different from that seen in carrier gas. In other experiments, the monolayers were treated with 0, 200, 500, and 1,000 uM hydrogen peroxide after equilibration with 0.4%, 1.7%, and 2.8% halothane or 0.7%, 2.8%, and 5% isoflurane in 5% carbon dioxide in air. Efficiency of replating was used to measure degree of injury. Both halothane and isoflurane enhance the sensitivity of the rat pulmonary arterial endothelial cell monolayers to injury by hydrogen peroxide. The sensitizing effect of halothane was reversed by removing the anesthetic. Halothane and isoflurane thus enhance rat pulmonary arterial endothelial cell sensitivity to injury by both hydrogen peroxide and phorbol myristate acetate stimulated neutrophils. In increasing rat pulmonary arterial endothelial cell sensitivity to injury by oxygen metabolites, halothane and isoflurane may be inhibiting processes involved in intracellular antioxidant defenses.
Shayevitz JR et al; Anesthesiol 74 (6): 1067-77 (1991)
Peripheral blood mononuclear cells from patients with halothane hepatitis are unusually susceptible to damage from phenytoin metabolites generated by an in vitro drug metabolising system. In order to provide more information about the nature of this susceptibility factor, the effect of removing calcium ions from the incubation medium of the test system was examined. Phenytoin metabolites were generated by incubating phenytoin with beta-naphthoflavone induced rat liver microsomes in the presence of 1,1,1-trichloropropene oxide, an epoxide hydrase inhibitor. When peripheral blood mononuclear cells from patients who had recovered from halothane hepatitis were incubated in this system and the maintained in calcium ion-containing tissue culture medium (without alpha-tocopherol) for 16 hr, cell death, as measured by trypan blue exclusion, was greatly increased (53% and 78% at 0.06 mmol/l and 0.12 mmol/l phenytoin, respectively) compared with control incubations (1,1,1-trichloropropene oxide omitted). Removal of calcium ions from the tissue culture medium effectively abolished reactive metabolite-induced cell death. Resting cytosolic free calcium ion concn in peripheral blood mononuclear cells was also measured using the quin-2 fluorescence method and total calcium ion content was measured by atomic absorption spectrometry. Although variability appeared greater among patients, mean values for these parameters among 12 patients with halothane hepatitis did not differ from controls. It is concluded that enhanced permeability of peripheral blood mononuclear cells to extracellular calcium ion may be an important factor in the pathogenesis of drug metabolite induced cell death in patients susceptible to halothane hepatitis. Such permeability to calcium ion is not evident in resting cells and presumably results from an interaction between electrophilic metabolites and the pumps which regulate cell calcium homeostasis.
Frost L et al; J Gastroenterol Hepatol 4 (1): 1-9 (1989)
For more Mechanism of Action (Complete) data for 2-BROMO-2-CHLORO-1,1,1-TRIFLUOROETHANE (7 total), please visit the HSDB record page.