



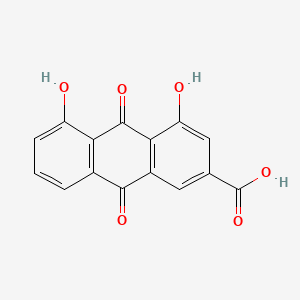

1. 1,8-dihydroxy-3-carboxyl-9,10-anthraquinone
2. 4,5-dihydroxyanthraquinone-2-carboxylic Acid
3. Dipropionyl Rhein
1. 478-43-3
2. Monorhein
3. Rhubarb Yellow
4. Rheic Acid
5. Cassic Acid
6. 4,5-dihydroxyanthraquinone-2-carboxylic Acid
7. Chrysazin-3-carboxylic Acid
8. 1,8-dihydroxy-3-carboxyanthraquinone
9. 4,5-dihydroxy-9,10-dioxo-9,10-dihydroanthracene-2-carboxylic Acid
10. Rheinic Acid
11. Nsc 38629
12. 1,8-dihydroxyanthraquinone-3-carboxylic Acid
13. 4,5-dihydroxy-9,10-dioxoanthracene-2-carboxylic Acid
14. 9,10-dihydro-4,5-dihydroxy-9,10-dioxo-2-anthracenecarboxylic Acid
15. 2-anthracenecarboxylic Acid, 9,10-dihydro-4,5-dihydroxy-9,10-dioxo-
16. Mfcd00009618
17. 9,10-dihydro-4,5-dihydroxy-9,10-dioxoanthracene-2-carboxylic Acid
18. Rhein (1,8-dihydroxy-3-carboxyl Anthraquinone)
19. Ym64c2p6ux
20. Mls000069639
21. Chebi:8825
22. 2-anthroic Acid, 9,10-dihydro-4,5-dihydroxy-9,10-dioxo-
23. 4,5-dihydroxy-2-anthraquinonecarboxylic Acid
24. 4,5-dioh-anthraquinone-2-cooh
25. Nsc-38629
26. Smr000058210
27. Dipropionyl Rhein
28. 1,8-dihydroxy-3-carboxyl Anthraquinone
29. 9,10-dihydro-4,5-dihydroxy-9,10-dioxo-2-anthroic Acid
30. Rhein(monorhein)
31. Ccris 5129
32. Einecs 207-521-4
33. Unii-ym64c2p6ux
34. Brn 2222155
35. Rhein - Monorhein
36. Rhn
37. Rhein,(s)
38. Rhein; Rhubarb Yellow
39. Rhein, Technical Grade
40. 4ie7
41. St057726
42. Regid855879
43. Rhein [inci]
44. Rhein-[13c6]
45. Dsstox_cid_6000
46. Rhein [mi]
47. Rhein, Analytical Standard
48. 1,8-dihydroxy-3-carboxyl-9,10-anthraquinone
49. Dsstox_rid_77984
50. Dsstox_gsid_26000
51. Schembl25253
52. Us9238626, Rhein
53. 4-10-00-04088 (beilstein Handbook Reference)
54. Cid_10168
55. Mls006011744
56. Chembl418068
57. Megxp0_001866
58. 4,5-dihydroxy-9,10-dioxo-anthracene-2-carboxylic Acid
59. Dtxsid4026000
60. Acon1_000217
61. Bdbm32021
62. Act03257
63. Bcp28202
64. Hy-n0105
65. Nsc38629
66. Zinc4098704
67. Tox21_201098
68. 4,5-dihydroxy-9,10-dioxo-9,10-dihydroanthracene-2-carboxylicacid
69. Bbl009695
70. Lmpk13040015
71. S2400
72. Stl141046
73. Akos005259272
74. Ac-7978
75. Cs-5239
76. Db13174
77. Ncgc00018199-01
78. Ncgc00018199-02
79. Ncgc00018199-03
80. Ncgc00018199-04
81. Ncgc00018199-05
82. Ncgc00018199-06
83. Ncgc00018199-07
84. Ncgc00023342-03
85. Ncgc00023342-04
86. Ncgc00258650-01
87. Am807834
88. As-11635
89. Cas-478-43-3
90. Sy051290
91. Db-014981
92. D3986
93. Ft-0645050
94. Ft-0674401
95. N1810
96. 4, 5-dihydroxyanthraquinone-2-carboxylic Acid
97. 2-anthraquinonecarboxylic Acid, 4,5-dihydroxy-
98. 78r433
99. Dihydroxy-3-carboxylanthraquinone, 1,8-
100. A827360
101. Q720356
102. Q-100512
103. 4,5-dihydroxy-9,10-dioxo-2-anthracenecarboxylic Acid
104. Brd-k27335680-001-01-9
105. 2-anthroic Acid,10-dihydro-4,5-dihydroxy-9,10-dioxo-
106. 4,5-dihydroxy-9,10-diketo-anthracene-2-carboxylic Acid
107. 2-anthracenecarboxylic Acid,10-dihydro-4,5-dihydroxy-9,10-dioxo-
108. 4,5-bis(oxidanyl)-9,10-bis(oxidanylidene)anthracene-2-carboxylic Acid
109. 4,5-dihydroxy-9,10-dioxo-9,10-dihydro-2-anthracenecarboxylic Acid #
110. 9,10-dihydro-4,5-dihydroxy-9,10-dioxo-2-anthracene Carboxylic Acid
111. 9,10-dihydro-4,5-dihydroxy-9,10-dioxo-2-anthracenecarboxylic Acid, 9ci
| Molecular Weight | 284.22 g/mol |
|---|---|
| Molecular Formula | C15H8O6 |
| XLogP3 | 2.2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 1 |
| Exact Mass | 284.03208797 g/mol |
| Monoisotopic Mass | 284.03208797 g/mol |
| Topological Polar Surface Area | 112 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 487 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
No approved indication.
**Liver: **Reverses animal models of non-alcoholic fatty liver disease by lowering liver lipids and reducing inflammation. Also reverses and prevents fibrosis in liver injury. **Kidney: **Protects against fibrosis in nephropathy models and improves epithelial tight junction function. **Bone and joint:** Decreases inflammation and cartilage destruction and also corrects altered osteoblast acitivity. **Lipid lowering and anti-obesity:** Reduces body weight and fat content, and lowers high density lipoprotein and low density lipoprotein. May prevent adipocyte differentiation. **Anti-oxidant/Pro-oxidant**: Reduces levels of reactive oxygen species (ROS) at concentrations of about 2-16 microM but induces the generation of ROS at concentrations of 50 microM and above. **Anti-cancer:** Rhein has been observed to produce DNA damage and suppress DNA repair in cancer cells. It induces apoptosis via ER stress, calcium, and mitochondria mediated pathways. Rhein also prevents cancer cell invasion into systemic circulation by preventing angiogenesis and breakdown of the extracellular matrix. Finally, rhein suppresses the activation of several tumor promoting signalling pathways. **Anti-inflammatory: ** Suppresses the production of pro-inflammatory cytokines such as interleukin-1beta and interleukin-6. **Anti-diabetic: **Lowers plasma glucose and increases survival of islet beta cells in type 2 diabetes mellitus models. **Anti-microbial:** Inhibits arylamine N-acteyltransferase and cell growth in Helicobacter pylori. Rhien also appears to be effective against many genotypes of Staphylococcus aureus. **Anti-allergenic:** Inhibits production of leukotrienes and the release of histamine from mast cells.
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Absorption
Tmax of 1.6-2.6 hours.
Route of Elimination
37% is excreted in urine and 53% in feces as estimated in rats.
Volume of Distribution
15-60L.
Clearance
Total CL is 1.5 L/h and renal CL is 0.1 L/h.
Metabolized primarily to rhein glucuronide and rhien sulfate.
4-10h.
**Liver: **The reversal of non-alcoholic fatty liver disease stems from rhien's lipid lowering and anti-obesity actions which result in an overall decrease in body weight, high density lipoprotein, and low density lipoprotein as well as its anti-inflammatory action. The reversal of hepatic fibrosis is thought to be due to rhien's anti-inflammatory and anti-oxidant action which suppresses the pro-fibrotic signalling from macrophages and further damage from reactive oxygen species respectively. Ultimately this results in reduced expression of alpha-smooth muscle actin (Alpha-SMA) which is indicative of decreased hepatic stellate cell and myofibroblast activation. Rhein also appears to suppress the expression of transforming growth factor-Beta (TGF-Beta) **Kidney: **The protection from fibrosis in the kidney also appears to stem from rhien's anti-inflammatory action resulting in less inflammatory cell infiltration along with suppression of alpha-SMA and fibronectin expression. These indicate a reduction in the activation of interstitial fibroblasts which are responsible for excess production of extracellular matrix components. Rhien may also suppress TGF-beta expression in the kidney. The anti-fibrotic mechanism of rhien may involve the upregulation of bone morphogenetic protein 7 and hepatic growth factor. In diabetic nephropathy rhein appears to suppress the expression of integrin-linked kinase leading to a reduction in the matrix metalloproteinase-9/tissue inhibitor of matrix metalloproteinase-1 ratio. The improvement of epithelial tight junction function seems to involve upregulation of zona occludins protein-1 and occludin expression. **Bone and joint:**Rhein reduces cartilage destruction by decreasing expression of matrix metalloproteinase (MMP)-1 and -3 as well as upregulating tissue inhibitor of matrix metalloproteinases which serve to reduce the activity of several MMPs. The anti-inflammatory action of rhein reduces the level of interleukin-1beta activity which plays a large role in reduction of extracellular matrix production, MMP activity, and continued inflammation. Rhein reduces abnormal osteoblast synthetic activity through an unknown mechanism. **Lipid lowering and anti-obesity: **Rhein is known to bind and inhibit liver X receptor alpha and beta with Kd values of 46.7 microM and 31.97 microM respectively. This decreases the expression of genes such as that of sterol regulatory element binding transcription factor 1 (SREBP1c) and its downstream genes for fatty acid synthase (FAS), steroyl-coenzyme A desaturase 1 (SCD1), and acetyl CoA carboxylase 1 (ACC1). SREBP1c, FAS, SCD1, and ACC1 are all involved in adipogenesis and their suppression results in less fat content. The genes for ABCA1 and ABCG1 are also suppressed. These correspond to cholesterol efflux trasporters and likely explain the reductiion in HDL and LDL seen with rhein. The inhibition of LXR by rhien relieves the inhibition on uncoupling protein 1 expression in brown adipose tissue. The result of this is increased thermogenesis which likely plays a role in the reduction of body weight produced by rhien. Additionally, rhein may downregulate peroxisome proliferator-receptor gamma and its downstream genes to inhibit adipocyte differentiation. **Anti-oxidant/Pro-oxidant:** The antoxidant mechanism is unknown. The pro-oxidant action of rhien may involve the inhibition of mitochondrial respiratory complex 1 and subsequent facilitation of NADH and NADPH dependent lipid peroxidation. **Anti-cancer:** The exact mechanism of rhein's ability to damage DNA and supress the expression of DNA repair enzymes ADR and MGMT is unknown. The mechanism through which rhien induces ER stress is unknown but likely involves its pro-oxidant properties. Rhein has been observed to produce increases in cytosolic calcium, reductions in mitochondrial membrane potential, and upregulation of pro-apoptotic proteins as well as leakage of cytochrome C which would induce apoptosis via the intrinsic pathway. The reduction of matrix metalloproteinase-9 serves to prevent extra cellular matrix breakdown by cancer cells and hinders their invasion into surrounding tissue. Rhein also decreases vascular endothelial growth factor expression through an unknown mechanism to prevent cancer cells from stimulating agiogenesis. Rhein reduces the activity of the nuclear factor kappa (NFkappaB) pathway by preventing the destruction of IKBalpha. The activity of the phosphoinositol 3-kinase/Akt pathway is also reduced by rhien. Rhein's inhibition of the mitogen-activated protein kinase pathways (particularly those involving extracellular signal regulated kinase) appears to follow a U-shaped dose response curve. ERK phosphorylation is inhibited at low concentrations of around 3microM but activated at higher concentrations of around 30microM. Furthermore, ERK phosporylation is again inhibited at extremely high concentrations in excess of 100 microM. The suppression of these three pathways is likely involved in the anti-proliferative effects of rhein. **Anti-inflammatory:** The mechanism of rhein's anti-inflammatory effect likely involves its inhibition of the NFkappa B pathway which plays a role in the production of many pro-inflammatory cytokines. Rhein's anti-oxidant activity may also play a role in preventing damage during inflammation. **Anti-diabetic:** Rhein is thought to increase islet beta cell survival by suppressing the expression of dynamin-related protein 1 and thereby preventing mitochondrial fission. Rhein's anti-oxidant properties are also thought to play a role in protecting islet beta cells. The reduction in plasma glucose is likely due to increased survival of islet beta cells and subsequent increases in insulin secretion. Rhein's anti-inflammatory action may also serve to reduce insulin resistance. **Anti-microbial:** Rhien inhibits H. pylori arylamine N-acetyltransferase in a dose dependent manner. The mechanism of rhein's anti-microbial effect on H. pylori and S. aureus are unknown. **Anti-allergenic:** Rhien inhibits 5-lipoxygenase with an IC50 of 13.7microM. Rhien also inhibits mast cell degranulation although the specific mechanism is unknown.