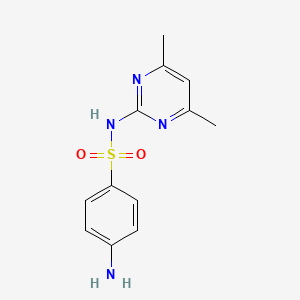

1. Benzenesulfonamide, 4-amino-n-(4,6-dimethyl-2-pyrimidinyl)-
2. Sulfadimezine
3. Sulfadimidine
4. Sulphamethazine
5. Sulphamezathine
1. Sulfadimidine
2. 57-68-1
3. Sulfadimerazine
4. Sulfamezathine
5. Sulphamethazine
6. Sulfadimethyldiazine
7. Sulfadimethylpyrimidine
8. 4-amino-n-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide
9. Sulphamezathine
10. Sulfadimezine
11. Sulfadimidin
12. Sulphadimidine
13. Sulphadimethylpyrimidine
14. Sulfadimesin
15. Sulfadimesine
16. Sulfadimezin
17. Sulfadimidinum
18. Sulfametazyny
19. Sulfamethiazine
20. Sulphamethasine
21. Sulphamidine
22. Sulphodimezine
23. Sulfadine
24. Cremomethazine
25. Sulfadimidina
26. Sulfametazina
27. Sulfodimezine
28. Azolmetazin
29. Dimezathine
30. Intradine
31. Kelametazine
32. Pirmazin
33. Spanbolet
34. Sulfodimesin
35. Superseptil
36. Superseptyl
37. Vertolan
38. Diazil
39. Mermeth
40. Neasina
41. Neazina
42. Sulfa-isodimerazine
43. Dimidin-r
44. Hava-span
45. 4,6-dimethyl-2-sulfanilamidopyrimidine
46. Calfspan Tablets
47. Sa Iii
48. 4-amino-n-(4,6-dimethyl-2-pyrimidinyl)benzenesulfonamide
49. Sulfamidine
50. 2-sulfanilamido-4,6-dimethylpyrimidine
51. Sulfasure Sr Bolus
52. N-(4,6-dimethyl-2-pyrimidyl)sulfanilamide
53. Benzenesulfonamide, 4-amino-n-(4,6-dimethyl-2-pyrimidinyl)-
54. Diazil (the Sulfanilamide)
55. Primazin
56. 2-(p-aminobenzenesulfonamido)-4,6-dimethylpyrimidine
57. 4-amino-n-(2,6-dimethyl-4-pyrimidinyl)benzenesulfonamide
58. A-502
59. 6-(4'-aminobenzol-sulfonamido)-2,4-dimethylpyrimidin
60. N(1)-(4,6-dimethyl-2-pyrimidyl)sulfanilamide
61. Nci-c56600
62. N(1)-(4,6-dimethyl-2-pyrimidinyl)sulfanilamide
63. Sulmet
64. Sulfadimidine;sulfadimerazine
65. (p-aminobenzolsulfonyl)-2-amino-4,6-dimethylpyrimidin
66. Sulfadimidine (inn)
67. Sulfamethazine (usp)
68. Sulfamethazine [usp]
69. 2-(4-aminobenzenesulfonamido)-4,6-dimethylpyrimidine
70. N(sup 1)-(4,6-dimethyl-2-pyrimidinyl)sulfanilamide
71. Chebi:102265
72. Sulfadimezinum
73. Nsc-67457
74. Nsc-683529
75. Sulfanilamide, N(sup1)-(4,6-dimethyl-2-pyrimidinyl)-
76. Mls000069711
77. 4-amino-n-(4,6-dimethyl-pyrimidin-2-yl)-benzenesulfonamide
78. Solfadimidina
79. N(sup1)-(2,6-dimethylpyrimid-4-yl)sulfanilamide
80. N(sup1)-(4,6-dimethyl-2-pyrimidyl)sulfanilamide
81. Nsc67457
82. Sulfadimidine-d4
83. Sulfanilamide, N1-(4,6-dimethyl-2-pyrimidinyl)-
84. Sulka S Boluses
85. N(sup1)-(4,6-dimethyl-2-pyrimidinyl)sulfanilamide
86. Nsc683529
87. 48u51w007f
88. 4,6-dimethylsulfadiazine
89. Bn-2409
90. Ncgc00018243-07
91. Smr000017409
92. Solfadimidina [dcit]
93. Sulfametazyny [polish]
94. Dsstox_cid_1290
95. Sulfamethazine 100 Microg/ml In Acetonitrile
96. Sulfadimidine [inn]
97. Dsstox_rid_76062
98. Dsstox_gsid_21290
99. Smz
100. Sulfadimidinum [inn-latin]
101. Sulfadimidina [inn-spanish]
102. Sulfametazina [italian]
103. Sulfadimidine [inn:ban]
104. Cas-57-68-1
105. Bn 2409
106. Ccris 3701
107. Sulfamezathine (tn)
108. Hsdb 4157
109. Einecs 200-346-4
110. Mfcd00006066
111. Nsc 67457
112. Brn 0261304
113. N(sup 1)-(4,6-dimethyl-2-pyrimidyl)sulfanilamide
114. Sulfamethazone
115. Diazilsulfadine
116. Calfspan
117. Panazin
118. Ai3-26817
119. Sulka K Boluses
120. S-dimidine
121. Dimidim-r
122. Unii-48u51w007f
123. 4-amino-n-(4,6-dimethylpyrimidin-2-yl)benzene-1-sulfonamide
124. (p-aminobenzolsulfonyl)-2-amino-4,6-dimethylpyrimidin [german]
125. 6-(4'-aminobenzol-sulfonamido)-2,4-dimethylpyrimidin [german]
126. Sulfadimidine,(s)
127. Sulfanilamide, N(1)-(4,6-dimethyl-2-pyrimidinyl)-
128. Sulfadimidine-13c6
129. 4-amino-n-(4,6-dimethyl-2-pyrimidyl)benzenesulfonamide
130. Sulfanilamide, N(sup1)-(2,6-dimethyl-4-pyrimidinyl)-
131. Sentry Aq Mardel Biospheres Maracyn Plus
132. Spectrum_000990
133. [(4-aminophenyl)sulfonyl](4,6-dimethylpyrimidin-2-yl)amine
134. 4-amino-n-(4
135. Opera_id_1374
136. Prestwick0_000775
137. Prestwick1_000775
138. Prestwick2_000775
139. Prestwick3_000775
140. Spectrum2_001321
141. Spectrum3_001700
142. Spectrum4_000344
143. Spectrum5_001270
144. Sulfamethazine, >=99%
145. Chembl446
146. Epitope Id:122238
147. Cambridge Id 5251384
148. Nciopen2_003489
149. Bidd:pxr0093
150. Oprea1_142608
151. Oprea1_677935
152. Bspbio_000850
153. Bspbio_003260
154. Cbdive_012932
155. Kbiogr_000747
156. Kbioss_001470
157. Sulfamethazine [hsdb]
158. Sulfamethazine [iarc]
159. 5-25-10-00250 (beilstein Handbook Reference)
160. Mls000103403
161. Mls001077331
162. Mls002454449
163. Divk1c_000293
164. Schembl151305
165. Spectrum1500548
166. Sulfadimidine [mart.]
167. Sulfamethazine [vandf]
168. Spbio_001441
169. Spbio_002789
170. Sulfadimidine [who-dd]
171. Sulfadimidine [who-ip]
172. Bpbio1_000936
173. Sulfamethazine [usp-rs]
174. Dtxsid6021290
175. Sulfanilamide, N(sup 1)-(4,6-dimethyl-2-pyrimidinyl)-
176. Aswvtgncazcnnr-uhfffaoysa-
177. Hms500o15
178. Kbio1_000293
179. Kbio2_001470
180. Kbio2_004038
181. Kbio2_006606
182. Kbio3_002480
183. Zinc57494
184. Ninds_000293
185. Hms1921a17
186. Hms2092i19
187. Hms3652k03
188. Pharmakon1600-01500548
189. Sulfadimidine [ep Impurity]
190. Sulfamethazine [green Book]
191. Albb-033473
192. Bcp28439
193. Hy-b0035
194. Sulfadimidine [ep Monograph]
195. Sulfadimidine For Peak Identification
196. Sulfamethazine [orange Book]
197. Tox21_110847
198. Tox21_202221
199. Tox21_303006
200. Ccg-39259
201. Nsc757326
202. S3133
203. Stk097514
204. Sulfadimidinum [who-ip Latin]
205. Sulfamethazine [usp Monograph]
206. Akos000119894
207. Sulfose Component Sulfamethazine
208. Tox21_110847_1
209. Db01582
210. Ms-1576
211. Nsc-757326
212. Idi1_000293
213. Terfonyl Component Sulfamethazine
214. Lantrisul Component Sulfamethazine
215. Ncgc00018243-01
216. Ncgc00018243-02
217. Ncgc00018243-03
218. Ncgc00018243-04
219. Ncgc00018243-05
220. Ncgc00018243-06
221. Ncgc00018243-08
222. Ncgc00018243-09
223. Ncgc00021490-03
224. Ncgc00021490-04
225. Ncgc00021490-05
226. Ncgc00021490-06
227. Ncgc00256371-01
228. Ncgc00259770-01
229. Sulfadimidine Solution, 1 Mg/ml In H2o
230. Sulfaloid Component Sulfamethazine
231. Wln: T6n Cnj Bmswr Dz& D1 F1
232. Ac-16126
233. Neotrizine Component Sulfamethazine
234. Sulfamethazine 100 Microg/ml In Methanol
235. Sulfamethazine Component Of Sulfose
236. Sulfanilamide,6-dimethyl-4-pyrimidinyl)-
237. Sbi-0051522.p003
238. Sulfamethazine (trisulfapyrimidines)
239. Sulfamethazine Component Of Terfonyl
240. Trisulfapyrimidines (sulfamethazine)
241. Sulfamethazine 1000 Microg/ml In Methanol
242. Sulfamethazine Component Of Lantrisul
243. Sulfamethazine Component Of Sulfaloid
244. Ft-0655603
245. Ft-0674743
246. N1-(4,6-dimethyl-2-pyrimidyl)sulfanilamide
247. Sulfamethazine Component Of Neotrizine
248. Sw219689-1
249. Benzenesulfonamide,6-dimethyl-4-pyrimidinyl)-
250. Triple Sulfoid Component Sulfamethazine
251. C19530
252. D02436
253. N1-(4,6-dimethyl-2-pyrimidinyl)sulfanilamide
254. Sulfamethazine 1000 Microg/ml In Acetonitrile
255. Ab00052097_12
256. Ab00052097_13
257. Sulfamethazine Component Of Triple Sulfoid
258. A831551
259. Sr-01000000211
260. Sulfamethazine, Vetec(tm) Reagent Grade, >=99%
261. Sulfamethazine, Vetranal(tm), Analytical Standard
262. Q3976823
263. Sr-01000000211-3
264. W-105450
265. 4-amino-n-(4,6-dimethyl-2-pyridyl)benzenesulfonamide
266. Brd-k11640013-001-02-6
267. Brd-k11640013-236-03-6
268. 2-(4-aminobenzenesulfonylamino)-4,6-dimethylpyrimidine
269. F1443-4796
270. Trisulfapyrimidines (sulfamethazine) [orange Book]
271. Sulfadimidine, European Pharmacopoeia (ep) Reference Standard
272. (4-amino-n-(4,6-dimethyl-2-pyrimidinyl)benzene Sulfonamide
273. 4-amino-n~1~-(4,6-dimethyl-2-pyrimidinyl)-1-benzenesulfonamide
274. Hsdb 4157; Hsdb 4157; Hsdb 4157;sulfadimidine;sulfadimerazine
275. Sulfamethazine, United States Pharmacopeia (usp) Reference Standard
276. Sulfadimidine For Peak Identification, European Pharmacopoeia (ep) Reference Standard
| Molecular Weight | 278.33 g/mol |
|---|---|
| Molecular Formula | C12H14N4O2S |
| XLogP3 | 0.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 278.08374688 g/mol |
| Monoisotopic Mass | 278.08374688 g/mol |
| Topological Polar Surface Area | 106 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 377 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Infective agents
National Library of Medicine's Medical Subject Headings. Sulfamethazine. Online file (MeSH, 2016). Available from, as of January 20, 2016: https://www.nlm.nih.gov/mesh/2016/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Sulfamethazine is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of March 23, 2016: https://clinicaltrials.gov/search/intervention=sulfamethazine
MEDICATION (VET): Sulfamethazine is used as a broad-spectrum antimicrobial to treat or prevent infections caused by susceptible organisms. Infections treated may include pneumonia, intestinal infections (especially coccidia), soft tissue infections and urinary tract infections (UTIs).
Papich, M.G. Saunders Handbook of Veterinary Drugs Small and Large Animal. 3rd ed. St. Louis, MO: Elsevier Saunders, 2011, p. 723
MEDICATION (VET): Sulfadimidine, which is also known as sulfamethazine, is widely used in veterinary medicine in combination with chlortetracycline and penicillin in pigs for maintenance of weight gain in the presence of atrophic rhinitis, growth promotion and increased feed efficiency. Sulfadimidine is also effective against a wide variety of diseases in food-producing animals. Common therapeutic uses in cattle include: treatment of bovine respiratory disease complex (shipping fever complex); necrotic pododermatitis (foot rot) and calf diphtheria; colibacillosis (bacterial scours); coccidiosis and acute mastitis and acute metritis. Common therapeutic uses in sheep include: treatment of pasteurellosis; bacteria pneumonia; colibacillosis (bacterial scours) and control and treatment of coccidiosis. Common therapeutic uses in pigs include: treatment of bacterial pneumonia; porcine colibacillosis (bacterial scours); bacterial swine enteritis; and reduction in the incidence of cervical abscesses. Common therapeutic uses in chickens include: control of infectious coryza; coccidiosis; acute fowl cholera; and pullorum disease. Common therapeutic uses in turkeys include: control of coccidiosis.
WHO/FAO; Thirty-fourth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); Toxicological Evaluation of Certain Veterinary Drug Residues in Food, WHO Food Additive Series 25: Sulfadimidine (1990). Available from, as of April 5, 2016: https://www.inchem.org/pages/jecfa.html
For more Therapeutic Uses (Complete) data for SULFAMETHAZINE (7 total), please visit the HSDB record page.
VET: Adverse effects associated with sulfonamides include allergic reactions, Type II and Type III hypersensitivity, arthropathy, anemia, thrombocytopenia, hepatopathy, hypothyroidism (with prolonged therapy), keratoconjunctivitis sicca, and skin reactions. Dogs may be more sensitive to sulfonamides than other animals because dogs lack the ability to acetylate sulfonamides to metabolites. Other, more toxic metabolites may persist. /Sulfonamides/
Papich, M.G. Saunders Handbook of Veterinary Drugs Small and Large Animal. 3rd ed. St. Louis, MO: Elsevier Saunders, 2011, p. 723
VET: Do not administer to animals with sensitivity to sulfonamides. Doberman pinschers may be more sensitive than other canine breeds to reactions from sulfonamides. Use cautiously in this breed. /Sulfonamides/
Papich, M.G. Saunders Handbook of Veterinary Drugs Small and Large Animal. 3rd ed. St. Louis, MO: Elsevier Saunders, 2011, p. 723
For the treatment bacterial infections causing bronchitis, prostatitis and urinary tract infections.
Sulfamethazine is a sulfonamide drug that inhibits bacterial synthesis of dihydrofolic acid by competing with para-aminobenzoic acid (PABA) for binding to dihydropteroate synthetase (dihydrofolate synthetase). Sulfamethazine is bacteriostatic in nature. Inhibition of dihydrofolic acid synthesis decreases the synthesis of bacterial nucleotides and DNA.
Anti-Infective Agents
Substances that prevent infectious agents or organisms from spreading or kill infectious agents in order to prevent the spread of infection. (See all compounds classified as Anti-Infective Agents.)
J01EB03
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
J01EB03
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01E - Sulfonamides and trimethoprim
J01EB - Short-acting sulfonamides
J01EB03 - Sulfadimidine
Absorption
Rapidly absorbed following oral administration.
The pharmacokinetics and metabolism of sulfadimidine (SDM) following intravenous administration of 100 mg/kg were studied in seven dwarf preruminant kids at 12 weeks of age, and again at the ruminant stage, when the animals were 18 weeks old. The persistence of SDM in 18-week-old kids was prolonged in comparison to the 12-week-old animals: a lower total body clearance and a prolonged elimination of SDM were obtained in the older animals. The renal clearance values of SDM and its metabolites were the same at both ages. The decrease of SDM clearance is related to the significant reduction in SDM hydroxylation at the older age. The reduced oxidative hepatic metabolism may result from the sexual maturation of the kids.
PMID:2704056 Nouws JF et al; J Vet Pharmacol Ther. 1989 Mar;12(1):19-24
Sulfamethazine acetylation phenotypes were determined in 19 healthy adults (aged 17-46 years; 15 men, four women; nine white, nine oriental, one black) given a single oral dose of 20 mg/kg bw sulfamethazine in 200 mL of water. The results showed a welldefined trimodal pattern for acetylation clearance and for overall elimination or metabolic rate constants and confirmed that the fast acetylator phenotype can be subdivided into intermediate and rapid acetylator groups. The average acetylation clearance rate for rapid acetylators (1.34 mL/min per kg bw) was 8.8 times the estimated clearance for slow acetylators (0.15 mL/min per kg bw) and 1.8 times that for intermediate acetylators (0.75 mL/min per kg bw). The average percentage of an absorbed dose excreted as acetylsulfamethazine in 72-hr urine was 93.7 for rapid acetylators, 87.7 for intermediate acetylators and 65.6 for slow acetylators.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V79 346-7 (2001)
The depletion of sulfadimidine (SDM) and its N4-acetyl and hydroxy metabolites was studied in eggs laid by hens after administration of either a single or multiple oral dosages of 100 mg SDM/kg. During medication and until 1 day after the last dose, the SDM and its metabolite concentrations in the egg white exceeded those in the egg yolk and reflected the plasma levels. In the period starting 2 days after the (last) dosage, the SDM concentration in the yolk became higher than in the egg white, and the drug depletion curves ran parallel. The mean maximum amount of SDM found in the whole egg was 1500 micrograms after a single and 1280 ug after multiple dosage. In eggs, traces of the N4-acetyl and 6-methylhydroxy metabolites could be detected (mainly in the egg white), and their concentrations were approximately 40 times lower than those of the parent drug. A highly significant correlation (P less than 0.005) was found between the development stage of the oocyte at the time of (last) medication and the amount of SDM found in the egg that developed from it. A period of 7 or 8 days after the (last) dosage of 100 mg SDM/kg/day is required to obtain SDM levels below 0.1 ug/g egg.
PMID:3564319 Geertsma MF et al; Vet Q 9 (1): 67-75 (1987)
Relatively strong blood-brain barrier to sulfamethazine was observed in rats. Passage of sulfamethazine from blood to brain was slow and difficult.
Siddique et al; Acta Vet Brno 48 (1-2-3-4): 79 (1980)
For more Absorption, Distribution and Excretion (Complete) data for SULFAMETHAZINE (12 total), please visit the HSDB record page.
Plasma disposition of sulfadimidine (SDM) and its metabolites was studied in laying hens after 100 mg SDM kg-1 doses were administered as a single intravenous dose, a single oral dose and multiple oral doses once daily for five consecutive days. SDM was extensively metabolized by acetylation and hydroxylation. In plasma, the metabolite observed with the highest concentration was N4-acetylsulfadimidine (N4-SDM) followed by hydroxymethylsulfadimidine (CH2OH) and 5-hydroxysulfadimidine. Following intravenous administration a biphasic elimination (as seen for a capacity limited reaction) pattern for SDM and its metabolites was observed. Multiple (5x) SDM dosing revealed plasma SDM concentrations ranging between 7 and 108 ug mL-1; within 96 hours of termination of the multiple SDM dosing, the plasma SDM concentration was below 0.01 ug mL-1. The renal clearances of N4-SDM and the hydroxy metabolites were approximately 10 times greater than that of SDM. The SDM mass balance (fecal/urinary recovery) showed a loss of 56 per cent after intravenous dosage and of 67 per cent after a single oral dosage; the hydroxy metabolites accounted for the highest percentage in feces/urine. Thus additional metabolic pathways must exist in laying hens.
PMID:3387673 Nouws JF et al; Res Vet Sci 44 (2): 202-7 (1988)
After 10 male and two female healthy volunteers were given oral doses of sulfamethazine of 12-17 mg/kg bw, 10-20% of the dose was excreted in the urine as free and conjugated hydroxylated metabolites and 61-81% as N4-acetylsulfamethazine. Six of the individuals were considered to be fast acetylators and six slow acetylators. The plasma concentration-time curve for sulfamethazine in the fast acetylators was biphasic, with half-times of 1.7 and 5.4 hr, respectively, whereas in the slow acetylators it was monophasic, with a half-time of 7.6 hr.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V79 347 (2001)
Sulfamethazine is metabolized similarly in animals and humans, with N4-acetylation dominating. A trimodal pattern of sulfamethazine acetylation is seen in humans. Differences in acetylation rates were observed between male and female rats and among females of different strains.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V79 348 (2001)
The pharmacokinetics of sulfamethizole, sulfamethoxazole, sulfadiazine, sulfapyridine and sulfadimidine have been studied in man. Renal clearance values of the metabolite N4-acetylsulphonamide are 6 to 20 times higher than those of the corresponding parent compound. The renal clearance of sulfonamides is dependent on the urine flow. N4-Acetylsulfonamide concentration-time profiles for plasma and urine have been constructed for the sulfonamides. The percentage N4-acetylsulfonamide-time profiles for plasma are excellent tools for establishing the acetylator phenotype, while those constructed from urine samples are less useful. Evidence is obtained that sulfadimidine is metabolically processes by 2 different isoenzymes, while sulfadiazine, sulfapyridine and sulfamethoxazole are processes by 1 acetylating isoenzyme. Sulfamethizole is acetylated to very little extent.
PMID:7389236 Vree TB et al; Clin Pharmacokinet 5 (3) :274-94 (1980)
For more Metabolism/Metabolites (Complete) data for SULFAMETHAZINE (9 total), please visit the HSDB record page.
After 10 male and two female healthy volunteers were given oral doses of sulfamethazine of 12-17 mg/kg bw, 10-20% of the dose was excreted in the urine as free and conjugated hydroxylated metabolites and 61-81% as N4-acetylsulfamethazine. Six of the individuals were considered to be fast acetylators and six slow acetylators. The plasma concentration-time curve for sulfamethazine in the fast acetylators was biphasic, with half-times of 1.7 and 5.4 hr, respectively, whereas in the slow acetylators it was monophasic, with a half-time of 7.6 hr.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V79 347 (2001)
Sulfadimidine is acetylated and hydroxylated in humans. ... The plasma concentration-time curve of sulfadimidine in fast acetylators is biphasic, with half-lives of 1.7 and 5.4 hr, whereas that in slow acetylators is monophasic, with a half-life of 7.6 hr. ...
PMID:3824429 Vree TB et al; Ther Drug Monit 8 (4): 434-9 (1986)
... /Following oral administration to swine,/ the mean half-life for sulfamethazine, the N4-glucose conjugate of sulfamethazine, and N4-acetylsulfamethazine was estimated to be 0.8 day. ...
PMID:2870889 Mitchell AD, Paulson GD; Drug Metab Dispos 14 (2): 161-5 (1986)
Sulfonamides inhibit the enzymatic conversion of pteridine and p-aminobenzoic acid (PABA) to dihydropteroic acid by competing with PABA for binding to dihydrofolate synthetase, an intermediate of tetrahydrofolic acid (THF) synthesis. THF is required for the synthesis of purines and dTMP and inhibition of its synthesis inhibits bacterial growth. Pyrimethamine and trimethoprim inhibit dihydrofolate reductase, another step in THF synthesis, and therefore act synergistically with the sulfonamides.