






1. Mln 9708
2. Mln-9708
3. Mln9708
4. Ninlaro
1. 1072833-77-2
2. Mln2238
3. Mln-2238
4. Mln 2238
5. Ixazomib [inn]
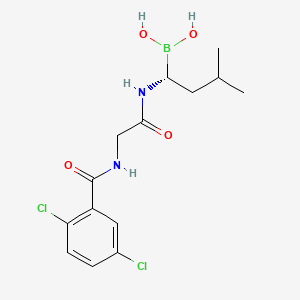
6. (r)-(1-(2-(2,5-dichlorobenzamido)acetamido)-3-methylbutyl)boronic Acid
7. (r)-1-(2-(2,5-dichlorobenzamido)acetamido)-3-methylbutylboronic Acid
8. Ixazomib (mln2238)
9. Mln-9708 Free Base
10. Chembl2141296
11. Ixazomib (usan)
12. Ixazomib [usan]
13. [(1r)-1-[[2-[(2,5-dichlorobenzoyl)amino]acetyl]amino]-3-methylbutyl]boronic Acid
14. [(1r)-1-{2-[(2,5-dichlorophenyl)formamido]acetamido}-3-methylbutyl]boronic Acid
15. Boronic Acid, B-((1r)-1-((2-((2,5-dichlorobenzoyl)amino)acetyl)amino)-3-methylbutyl)-
16. 71050168a2
17. Ixazomib [usan:inn]
18. Ixozamib
19. Ixazomib Impurity
20. Unii-71050168a2
21. Ixazomib(mln2238)
22. Mln2238(ixazomib)
23. Ixazomib [mi]
24. Ixazomib [who-dd]
25. Ixazomib (mln-2238)
26. Gtpl8450
27. Schembl3742758
28. Chebi:90942
29. Ex-a547
30. Dtxsid701025662
31. Amy19380
32. Bcp02410
33. Bcp24078
34. Bdbm50398609
35. Mfcd18251438
36. Nsc766907
37. S2180
38. Akos015995120
39. Zinc169946773
40. Bcp9000953
41. Ccg-264938
42. Cs-1657
43. Db09570
44. Nsc-766907
45. 1072833-77-2 (free)
46. Ncgc00249611-01
47. Ncgc00249611-03
48. Ncgc00249611-04
49. Ac-28456
50. As-55976
51. Hy-10453
52. Sw219743-1
53. J3.602.191h
54. A25328
55. D10130
56. J-001749
57. Brd-k78659596-001-01-3
58. Q20948663
59. N-[(1r)-1-borono-3-methylbutyl]-n(2)-(2,5-dichlorobenzoyl)glycinamide
60. ((1r)-1-((2,5-dichlorobenzamido)acetamido)-3-methylbutyl)boronic Acid
61. (r)-1-(2-(2,5-dichlorobenzamido)acetamido)-3-methylbutylboronic Acid;ixazomib
62. [(1r)-1-[[2-[(2,5-dichlorobenzoyl)amino]acetyl]amino]-3-methyl-butyl]boronic Acid
| Molecular Weight | 361.0 g/mol |
|---|---|
| Molecular Formula | C14H19BCl2N2O4 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 7 |
| Exact Mass | 360.0814926 g/mol |
| Monoisotopic Mass | 360.0814926 g/mol |
| Topological Polar Surface Area | 98.7 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 412 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Ixazomib is indicated in combination with lenalidomide and dexamethasone for the treatment of patients with multiple myeloma who have received at least one prior therapy.
FDA Label
Treatment of lymphoid malignancies (excluding multiple myeloma)
In vitro studies have shown ixazomib to induce apoptosis in multiple myeloma cells sensitive or resistant to other conventional therapies. In mouse xenograft models, ixazomib induced tumor growth inhibition.
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Protease Inhibitors
Compounds which inhibit or antagonize biosynthesis or actions of proteases (ENDOPEPTIDASES). (See all compounds classified as Protease Inhibitors.)
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01X - Other antineoplastic agents
L01XG - Proteasome inhibitors
L01XG03 - Ixazomib
Absorption
After oral administration, the time to reach maximum concentration in plasma was 1 hour. The mean absolute oral bioavailability is 58%.
Route of Elimination
62% in urine and 22% in feces.
Volume of Distribution
The steady-state volume of distribution is 543 L.
Metabolism of ixazomib is expected to be by CYP and non-CYP pathways, with no predominant CYP isozyme contribution. At higher than clinical concentrations, ixazomib was metabolized by multiple CYP isoforms with estimated relative contributions of 3A4 (42%), 1A2 (26%), 2B6 (16%), 2C8 (6%), 2D6 (5%), 2C19 (5%) and 2C9 (<1%).
Terminal half-life is 9.5 days.
Ixazomib is an N-capped dipeptidyl leucine boronic acid which reversibly inhibits the CT-L proteolytic (5) site of the 20S proteasome. At higher concentrations, ixazomib also seems to inhibit the proteolytic 1 and 2 subunits and to induce accumulation of ubiquitinated proteins.
