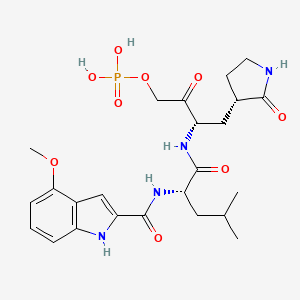

1. ((3s)-3-((2s)-2-((4-methoxy-1h-indol-2-yl)formamido)-4-methylpentanamido)-2-oxo-4-((3s)-2-oxopyrrolidin-3-yl)butoxy)phosphonic Acid
2. (14c)-pf-07304814
3. 14c Labeled Pf-07304814
4. 14c-pf-07304814
5. 4-methoxy-n-((2s)-4-methyl-1-oxo-1-(((2s)-3-oxo-1-((3s)-2-oxopyrrolidin-3-yl)-4-(phosphonooxy)butan-2-yl)amino)pentan-2-yl)-1h-indole-2-carboxamide
6. C14-pf-07304814
7. N-((1s)-1-((((1s)-3-hydroxy-2-oxo-1-(((3s)-2-oxopyrrolidin-3-yl)methyl)propyl)amino)carbonyl)-3-methylbutyl)-4-methoxy-1h-indole-2-carboxamide
8. Pf-00835231
9. Pf-07304814
10. Pf-835231
1. Pf-07304814
2. Lufotrelvir [usan]
3. Xj51yob1sc
4. Pf07304814
5. 2468015-78-1
6. (3s)-3-({n-[(4-methoxy-1h-indol-2-yl)carbonyl]-l-leucyl}amino)-2-oxo-4-[(3s)-2-oxopyrrolidin-3-yl]butyl Dihydrogen Phosphate
7. [(3~{s})-3-[[(2~{s})-2-[(4-methoxy-1~{h}-indol-2-yl)carbonylamino]-4-methyl-pentanoyl]amino]-2-oxidanylidene-4-[(3~{r})-2-oxidanylidene-3,4-dihydropyrrol-3-yl]butyl] Dihydrogen Phosphate
8. 1h-indole-2-carboxamide, 4-methoxy-n-((1s)-3-methyl-1-((((1s)-2-oxo-1-(((3s)-2-oxo-3-pyrrolidinyl)methyl)-3-(phosphonooxy)propyl)amino)carbonyl)butyl)-
9. 4-methoxy-n-((2s)-4-methyl-1-oxo-1-(((2s)-3-oxo-1-((3s)-2-oxopyrrolidin-3-yl)-4-(phosphonooxy)butan-2-yl)amino)pentan-2-yl)-1h-indole-2-carboxamide
10. Unii-xj51yob1sc
11. Lufotrelvir [inn]
12. Chembl4802214
13. Gtpl11249
14. Chebi:173073
15. Bdbm510049
16. Dtxsid501337108
17. Ex-a4702
18. Who 12095
19. Pf-07304814 [who-dd]
20. Hy-138078
21. Cs-0144500
22. Pf 07304814
23. (3s)-3-{[(2s)-2-{[(4-methoxy-1h-indol-2-yl)carbonyl]amino}-4-methylpentanoyl]amino}-2-oxo-4-[(3s)-2-oxopyrrolidin-3-yl]butyl Dihydrogen Phosphate
24. [(3s)-3-[(2s)-2-[(4-methoxy-1h-indol-2-yl)formamido]-4-methylpentanamido]-2-oxo-4-[(3s)-2-oxopyrrolidin-3-yl]butoxy]phosphonic Acid
| Molecular Weight | 552.5 g/mol |
|---|---|
| Molecular Formula | C24H33N4O9P |
| XLogP3 | 0.5 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 13 |
| Exact Mass | 552.19851564 g/mol |
| Monoisotopic Mass | 552.19851564 g/mol |
| Topological Polar Surface Area | 196 Ų |
| Heavy Atom Count | 38 |
| Formal Charge | 0 |
| Complexity | 927 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
The structure of PF-07304814 contains a phosphate group, allowing the compound to be soluble and then subsequently cleaved in tissue by alkaline phosphatase. This step allows for the release of PF-00835231, which is the compound that exerts anti-viral activity against the 3CL protease of SARS-CoV-2. The active compound, PF-00835231, was shown to have potent and broad-spectrum inhibitory activity against numerous coronavirus 3CL proteases.
