
1. 2,6-piperidinedione, 3-(4-amino-1,3-dihydro-1-oxo-2h- Isoindol-2-yl)-
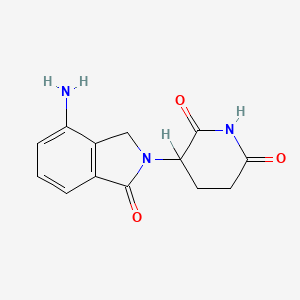
2. 3-(4-amino-1-oxoisoindolin-2-yl)piperidine-2,6-dione
3. Cc 5013
4. Cc-5013
5. Cc5013
6. Imid3 Cpd
7. Revimid
8. Revlimid
1. 191732-72-6
2. Revlimid
3. Revimid
4. 3-(4-amino-1-oxoisoindolin-2-yl)piperidine-2,6-dione
5. Cc-5013
6. Lenalidomide (cc-5013)
7. Cdc 501
8. Cdc-501
9. Imid3
10. 3-(4-amino-1-oxo-1,3-dihydro-2h-isoindol-2-yl)piperidine-2,6-dione
11. 3-(7-amino-3-oxo-1h-isoindol-2-yl)piperidine-2,6-dione
12. Cc 5013
13. Lenadoamide
14. 2,6-piperidinedione, 3-(4-amino-1,3-dihydro-1-oxo-2h-isoindol-2-yl)-
15. Revlimid (tn)
16. Nsc-747972
17. Chebi:63791
18. C13h13n3o3
19. F0p408n6v4
20. Syp-1512
21. Mfcd07772307
22. 1-oxo-2-(2,6-dioxopiperidin-3-yl)-4-aminoisoindoline
23. Ncgc00167491-01
24. Lenalidomide [usan]
25. 3-(4-amino-1-oxo-2,3-dihydro-1h-isoindol-2-yl)piperidine-2,6-dione
26. Dsstox_cid_26664
27. Dsstox_rid_81806
28. Dsstox_gsid_46664
29. Revlimid (lenalidomide)
30. 3-(4-amino-1-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione
31. Cas-191732-72-6
32. (3s)-3-(4-amino-1-oxo-1,3-dihydro-2h-isoindol-2-yl)piperidine-2,6-dione
33. Lenalidomide (usan/inn)
34. Unii-f0p408n6v4
35. Lenalidomide [usan:inn:ban]
36. Enmd 0997
37. Enmd-0997
38. Hsdb 8220
39. Imid-5013
40. Cdc-5013
41. Albb-015321
42. Lenalidomide [mi]
43. Lenalidomide [inn]
44. Lenalidomide [jan]
45. Chembl848
46. Revlimid (tn) (celgene)
47. Lenalidomide [vandf]
48. Schembl32978
49. Lenalidomide [mart.]
50. Mls003899194
51. Lenalidomide [who-dd]
52. Gtpl7331
53. Schembl1980410
54. Lenalidomide [ema Epar]
55. 3-(4-amino-1-oxo-2-isoindolinyl)piperidine-2,6-dione
56. Dtxsid8046664
57. Bdbm65454
58. 3-(7-amino-3-oxo-1h-isoindol-2-yl)-piperidine-2,6-dione
59. Bcpp000186
60. Hms3654g07
61. Hms3674c05
62. Lenalidomide [orange Book]
63. Bcp01390
64. Hy-a0003
65. Revlimid, Lenalidomide, Cc-5013
66. Tox21_112492
67. Ac-914
68. Nsc747972
69. S1029
70. Stk639603
71. 2,6-piperidinedione, 3-(4-amino-1,3-dihydro-1-oxo-2h- Isoindol-2-yl)-
72. Akos005146276
73. Akos005174869
74. Tox21_112492_1
75. Bcp9000847
76. Ccg-264781
77. Cs-0125
78. Db00480
79. Ks-1207
80. Nsc 747972
81. Sb66166
82. Ncgc00167491-02
83. Ncgc00167491-03
84. Ncgc00167491-04
85. 443912-14-9
86. Bl164614
87. Bp-27972
88. Smr002529986
89. Sy047646
90. Am20050439
91. Ft-0659651
92. Ft-0670758
93. Ft-0670759
94. Sw218084-2
95. Ec-000.2340
96. D04687
97. Ab01273975-01
98. Ab01273975-02
99. Ab01273975_03
100. 732l726
101. Q425681
102. Sr-01000883999
103. Q-101410
104. Sr-01000883999-1
105. Z1741976709
106. 2, 3-(4-amino-1,3-dihydro-1-oxo-2h-isoindol-2-yl)-
107. 3-(4-amino-1,3-dihydro-1-oxo-2h-isoindol-2-yl)-2,6-piperidinedione
108. 3-(4-amino-1-oxo-1,3-dihydro-2h-isoindol-2-yl)-2,6-dioxopiperidine
109. 3-(4-amino-1-oxo-1,3-dihydro-2h-isoindol-2-yl)-2,6-piperidinedione
110. 4-amino-2-(6-hydroxy-2-oxo-2,3,4,5-tetrahydropyridin-3-yl)-2,3-dihydro-1h-isoindol-1-one
1. Lenalidomide Hcl
2. 1243329-97-6
3. Lenalidomide Hydrochloride
| Molecular Weight | 259.26 g/mol |
|---|---|
| Molecular Formula | C13H13N3O3 |
| XLogP3 | -0.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | 259.09569129 g/mol |
| Monoisotopic Mass | 259.09569129 g/mol |
| Topological Polar Surface Area | 92.5 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 437 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Revlimid |
| PubMed Health | Lenalidomide (By mouth) |
| Drug Classes | Immune Modulator |
| Drug Label | REVLIMID, a thalidomide analogue, is an immunomodulatory agent with antiangiogenic and antineoplastic properties. The chemical name is 3-(4-amino-1-oxo 1,3-dihydro-2H-isoindol-2-yl) piperidine-2,6-dione and it has the following chemical structure: 3-... |
| Active Ingredient | Lenalidomide |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 2.5mg; 25mg; 15mg; 5mg; 10mg; 20mg |
| Market Status | Prescription |
| Company | Celgene |
| 2 of 2 | |
|---|---|
| Drug Name | Revlimid |
| PubMed Health | Lenalidomide (By mouth) |
| Drug Classes | Immune Modulator |
| Drug Label | REVLIMID, a thalidomide analogue, is an immunomodulatory agent with antiangiogenic and antineoplastic properties. The chemical name is 3-(4-amino-1-oxo 1,3-dihydro-2H-isoindol-2-yl) piperidine-2,6-dione and it has the following chemical structure: 3-... |
| Active Ingredient | Lenalidomide |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 2.5mg; 25mg; 15mg; 5mg; 10mg; 20mg |
| Market Status | Prescription |
| Company | Celgene |
Angiogenesis Inhibitors; Immunologic Factors
National Library of Medicine's Medical Subject Headings. Lenalidomide. Online file (MeSH, 2014). Available from, as of December 18, 2014: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
Revlimid in combination with dexamethasone is indicated for the treatment of patients with multiple myeloma (MM) who have received at least one prior therapy. /Included in US product label/
NIH; DailyMed. Current Medication Information for Revlimid (Lenalidomide) Capsule (Revised: September 2014). Available from, as of February 23, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5fa97bf5-28a2-48f1-8955-f56012d296be
Revlimid is indicated for the treatment of patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes (MDS) associated with a deletion 5q cytogenetic abnormality with or without additional cytogenetic abnormalities. /Included in US product label/
NIH; DailyMed. Current Medication Information for Revlimid (Lenalidomide) Capsule (Revised: September 2014). Available from, as of December 31, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5fa97bf5-28a2-48f1-8955-f56012d296be
Revlimid is indicated for the treatment of patients with mantle cell lymphoma (MCL) whose disease has relapsed or progressed after two prior therapies, one of which included bortezomib. /Included in US product label/
NIH; DailyMed. Current Medication Information for Revlimid (Lenalidomide) Capsule (Revised: September 2014). Available from, as of December 31, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5fa97bf5-28a2-48f1-8955-f56012d296be
For more Therapeutic Uses (Complete) data for Lenalidomide (7 total), please visit the HSDB record page.
/BOXED WARNING/ WARNING: EMBRYO-FETAL TOXICITY. Do not use Revlimid during pregnancy. Lenalidomide, a thalidomide analogue, caused limb abnormalities in a developmental monkey study. Thalidomide is a known human teratogen that causes severe life-threatening human birth defects. If lenalidomide is used during pregnancy, it may cause birth defects or embryo-fetal death. In females of reproductive potential, obtain 2 negative pregnancy tests before starting Revlimid treatment. Females of reproductive potential must use 2 forms of contraception or continuously abstain from heterosexual sex during and for 4 weeks after Revlimid treatment. To avoid embryo-fetal exposure to lenalidomide, Revlimid is only available through a restricted distribution program, the Revlimid REMS program (formerly known as the RevAssist program).
NIH; DailyMed. Current Medication Information for Revlimid (Lenalidomide) Capsule (Revised: September 2014). Available from, as of December 31, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5fa97bf5-28a2-48f1-8955-f56012d296be
/BOXED WARNING/ WARNING: HEMATOLOGIC TOXICITY. Revlimid can cause significant neutropenia and thrombocytopenia. Eighty percent of patients with del 5q myelodysplastic syndromes had to have a dose delay/reduction during the major study. Thirty-four percent of patients had to have a second dose delay/reduction. Grade 3 or 4 hematologic toxicity was seen in 80% of patients enrolled in the study. Patients on therapy for del 5q myelodysplastic syndromes should have their complete blood counts monitored weekly for the first 8 weeks of therapy and at least monthly thereafter. Patients may require dose interruption and/or reduction. Patients may require use of blood product support and/or growth factors
NIH; DailyMed. Current Medication Information for Revlimid (Lenalidomide) Capsule (Revised: September 2014). Available from, as of February 27, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5fa97bf5-28a2-48f1-8955-f56012d296be
/BOXED WARNING/ WARNING: VENOUS AND ARTERIAL THROMBOEMBOLISM. Revlimid has demonstrated a significantly increased risk of deep vein thrombosis (DVT) and pulmonary embolism (PE), as well as risk of myocardial infarction and stroke in patients with multiple myeloma who were treated with Revlimid and dexamethasone therapy. Monitor for and advise patients about signs and symptoms of thromboembolism. Advise patients to seek immediate medical care if they develop symptoms such as shortness of breath, chest pain, or arm or leg swelling. Thromboprophylaxis is recommended and the choice of regimen should be based on an assessment of the patient's underlying risks.
NIH; DailyMed. Current Medication Information for Revlimid (Lenalidomide) Capsule (Revised: September 2014). Available from, as of February 27, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5fa97bf5-28a2-48f1-8955-f56012d296be
Patients with multiple myeloma treated with lenalidomide in studies including melphalan and stem cell transplantation had a higher incidence of second primary malignancies, particularly acute myelogenous leukemia (AML) and Hodgkin lymphoma, compared to patients in the control arms who received similar therapy but did not receive lenalidomide. Monitor patients for the development of second malignancies. Take into account both the potential benefit of lenalidomide and the risk of second primary malignancies when considering treatment with lenalidomide.
NIH; DailyMed. Current Medication Information for Revlimid (Lenalidomide) Capsule (Revised: September 2014). Available from, as of February 27, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5fa97bf5-28a2-48f1-8955-f56012d296be
For more Drug Warnings (Complete) data for Lenalidomide (19 total), please visit the HSDB record page.
Lenalidomide is indicated for the treatment of adult patients with multiple myeloma (MM) in combination with dexamethasone. It is also indicated as maintenance therapy in multiple myeloma following autologous hematopoietic stem cell transplantation (auto-HSCT). It is indicated for the treatment of adult patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes (MDS) associated with a deletion 5q cytogenetic abnormality with or without additional cytogenetic abnormalities. Lenalidomide is indicated for the treatment of adult patients with mantle cell lymphoma (MCL) whose disease has relapsed or progressed after two prior therapies, one of which included bortezomib. In combination with a rituximab product, lenalidomide is indicated for the treatment of adult patients with previously treated follicular lymphoma (FL) or previously treated marginal zone lymphoma (MZL).
FDA Label
* Multiple myeloma:
Revlimid as monotherapy is indicated for the maintenance treatment of adult patients with newly diagnosed multiple myeloma who have undergone autologous stem cell transplantation.
Revlimid as combination therapy with dexamethasone, or bortezomib and dexamethasone, or melphalan and prednisone (see section 4. 2) is indicated for the treatment of adult patients with previously untreated multiple myeloma who are not eligible for transplant.
Revlimid in combination with dexamethasone is indicated for the treatment of multiple myeloma in adult patients who have received at least one prior therapy.
* Myelodysplastic syndromes:
Revlimid as monotherapy is indicated for the treatment of adult patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with an isolated deletion 5q cytogenetic abnormality when other therapeutic options are insufficient or inadequate.
* Mantle cell lymphoma:
Revlimid as monotherapy is indicated for the treatment of adult patients with relapsed or refractory mantle cell lymphoma.
* Follicular lymphoma:
Revlimid in combination with rituximab (anti-CD20 antibody) is indicated for the treatment of adult patients with previously treated follicular lymphoma (Grade 1 3a).
* Multiple myeloma:
Lenalidomide Accord as monotherapy is indicated for the maintenance treatment of adult patients with newly diagnosed multiple myeloma who have undergone autologous stem cell transplantation.
Lenalidomide Accord as combination therapy with dexamethasone, or bortezomib and dexamethasone, or melphalan and prednisone (see section 4. 2) is indicated for the treatment of adult patients with previously untreated multiple myeloma who are not eligible for transplant.
Lenalidomide Accord in combination with dexamethasone is indicated for the treatment of multiple myeloma in adult patients who have received at least one prior therapy.
* Follicular lymphoma:
Lenalidomide Accord in combination with rituximab (anti-CD20 antibody) is indicated for the treatment of adult patients with previously treated follicular lymphoma (Grade 1 3a).
Treatment of mature B-cell neoplasms
Treatment of diffuse large B-cell lymphoma
Myelodysplastic Syndrome
* Multiple myeloma:
Lenalidomide Mylan as monotherapy is indicated for the maintenance treatment of adult patients with newly diagnosed multiple myeloma who have undergone autologous stem cell transplantation.
Lenalidomide Mylan as combination therapy with dexamethasone, or bortezomib and dexamethasone, or melphalan and prednisone is indicated for the treatment of adult patients with previously untreated multiple myeloma who are not eligible for transplant.
Lenalidomide Mylan in combination with dexamethasone is indicated for the treatment of multiple myeloma in adult patients who have received at least one prior therapy.
* Follicular lymphoma:
Lenalidomide Mylan in combination with rituximab (anti-CD20 antibody) is indicated for the treatment of adult patients with previously treated follicular lymphoma (Grade 1-3a).
* Multiple myeloma:
Lenalidomide krka d. d. Novo mesto as monotherapy is indicated for the maintenance treatment of adult patients with newly diagnosed multiple myeloma who have undergone autologous stem cell transplantation.
Lenalidomide krka d. d. Novo mesto as combination therapy with dexamethasone, or bortezomib and dexamethasone, or melphalan and prednisone (see section 4. 2) is indicated for the treatment of adult patients with previously untreated multiple myeloma who are not eligible for transplant.
Lenalidomide krka d. d. Novo mesto in combination with dexamethasone is indicated for the treatment of multiple myeloma in adult patients who have received at least one prior therapy.
* Follicular lymphoma:
Lenalidomide krka d. d. Novo mesto in combination with rituximab (anti-CD20 antibody) is indicated for the treatment of adult patients with previously treated follicular lymphoma (Grade 1 3a).
* Multiple myeloma:
Lenalidomide krka d. d. Novo mesto as monotherapy is indicated for the maintenance treatment of adult patients with newly diagnosed multiple myeloma who have undergone autologous stem cell transplantation.
Lenalidomide krka d. d. Novo mesto as combination therapy with dexamethasone, or bortezomib and dexamethasone, or melphalan and prednisone (see section 4. 2) is indicated for the treatment of adult patients with previously untreated multiple myeloma who are not eligible for transplant.
Lenalidomide krka d. d. Novo mesto in combination with dexamethasone is indicated for the treatment of multiple myeloma in adult patients who have received at least one prior therapy.
* Follicular lymphoma:
Lenalidomide krka d. d. Novo mesto in combination with rituximab (anti-CD20 antibody) is indicated for the treatment of adult patients with previously treated follicular lymphoma (Grade 1 3a).
* Multiple myeloma:
Lenalidomide Krka as monotherapy is indicated for the maintenance treatment of adult patients with newly diagnosed multiple myeloma who have undergone autologous stem cell transplantation.
Lenalidomide Krka as combination therapy with dexamethasone, or bortezomib and dexamethasone, or melphalan and prednisone (see section 4. 2) is indicated for the treatment of adult patients with previously untreated multiple myeloma who are not eligible for transplant.
Lenalidomide Krka in combination with dexamethasone is indicated for the treatment of multiple myeloma in adult patients who have received at least one prior therapy.
* Myelodysplastic syndromes:
Lenalidomide Krka as monotherapy is indicated for the treatment of adult patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with an isolated deletion 5q cytogenetic abnormality when other therapeutic options are insufficient or inadequate.
Mantle cell lymphoma
Lenalidomide Krka as monotherapy is indicated for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (see sections 4. 4 and 5. 1).
* Follicular lymphoma:
Lenalidomide Krka in combination with rituximab (anti-CD20 antibody) is indicated for the treatment of adult patients with previously treated follicular lymphoma (Grade 1 3a).
* Multiple myeloma:
Lenalidomide Krka as monotherapy is indicated for the maintenance treatment of adult patients with newly diagnosed multiple myeloma who have undergone autologous stem cell transplantation.
Lenalidomide Krka as combination therapy with dexamethasone, or bortezomib and dexamethasone, or melphalan and prednisone (see section 4. 2) is indicated for the treatment of adult patients with previously untreated multiple myeloma who are not eligible for transplant.
Lenalidomide Krka in combination with dexamethasone is indicated for the treatment of multiple myeloma in adult patients who have received at least one prior therapy.
* Myelodysplastic syndromes:
Lenalidomide Krka as monotherapy is indicated for the treatment of adult patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with an isolated deletion 5q cytogenetic abnormality when other therapeutic options are insufficient or inadequate.
* Mantle cell lymphoma:
Lenalidomide Krka as monotherapy is indicated for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (see sections 4. 4 and 5. 1).
* Follicular lymphoma:
Lenalidomide Krka in combination with rituximab (anti-CD20 antibody) is indicated for the treatment of adult patients with previously treated follicular lymphoma (Grade 1 3a).
* Multiple myeloma:
Lenalidomide Krka d. d. as monotherapy is indicated for the maintenance treatment of adult patients with newly diagnosed multiple myeloma who have undergone autologous stem cell transplantation.
Lenalidomide Krka d. d. as combination therapy with dexamethasone, or bortezomib and dexamethasone, or melphalan and prednisone (see section 4. 2) is indicated for the treatment of adult patients with previously untreated multiple myeloma who are not eligible for transplant.
Lenalidomide Krka d. d. in combination with dexamethasone is indicated for the treatment of multiple myeloma in adult patients who have received at least one prior therapy.
* Myelodysplastic syndromes:
Lenalidomide Krka d. d. as monotherapy is indicated for the treatment of adult patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with an isolated deletion 5q cytogenetic abnormality when other therapeutic options are insufficient or inadequate.
* Follicular lymphoma:
Lenalidomide Krka d. d. in combination with rituximab (anti-CD20 antibody) is indicated for the treatment of adult patients with previously treated follicular lymphoma (Grade 1 3a).
In hematological malignancies, the immune system is deregulated in the form of altered cytokine networks in the tumour microenvironment, defective T cell regulation of host-tumour immune interactions, and diminished NK cell activity. Lenalidomide is an immunomodulatory agent with antineoplastic, antiangiogenic, and anti-inflammatory properties. Lenalidomide exerts direct cytotoxicity by increasing apoptosis and inhibiting the proliferation of hematopoietic malignant cells. It delays tumour growth in nonclinical hematopoietic tumour models _in vivo_, including multiple myeloma. Lenalidomide also works to limit the invasion or metastasis of tumour cells and inhibits angiogenesis. Lenalidomide also mediates indirect antitumour effects via its immunomodulatory actions: it inhibits the production of pro-inflammatory cytokines, which are implicated in various hematologic malignancies. Lenalidomide enhances the host immunity by stimulating T cell proliferation and enhancing the activity of natural killer (NK) cells. Lenalidomide is about 1001000 times more potent in stimulating T cell proliferation than [thalidomide]. _In vitro_, it enhances antibody-dependent cell-mediated cytotoxicity (ADCC), which is even more pronounced when used in combination with rituximab. Due to its anti-inflammatory properties, lenalidomide has been investigated in the context of inflammatory and autoimmune diseases, such as amyotrophic lateral sclerosis.
Immunologic Factors
Biologically active substances whose activities affect or play a role in the functioning of the immune system. (See all compounds classified as Immunologic Factors.)
Angiogenesis Inhibitors
Agents and endogenous substances that antagonize or inhibit the development of new blood vessels. (See all compounds classified as Angiogenesis Inhibitors.)
L04AX04
L04AX04
L04AX07
L04AX04
L04AX04
L04AX04
L04AX04
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L04 - Immunosuppressants
L04A - Immunosuppressants
L04AX - Other immunosuppressants
L04AX04 - Lenalidomide
Absorption
Following oral administration, lenalidomide is rapidly absorbed with high bioavailability. It has a Tmax ranging from 0.5 to six hours. Lenalidomide exhibits a linear pharmacokinetic profile, with its AUC and Cmax increasing proportionally with dose. Multiple dosing does not result in drug accumulation. In healthy male subjects, the Cmax was 413 77 ng/ml and the AUCinfinity was 1319 162 h x ng/ml.
Route of Elimination
Lenalidomide is eliminated predominantly via urinary excretion in the unchanged form. Following oral administration of 25 mg of radiolabeled lenalidomide in healthy subjects, about 90% of the dose (4.59% as metabolites) was eliminated in urine and 4% of the dose (1.83% as metabolites) was eliminated in feces within ten days post-dose. Approximately 85% of the dose was excreted as lenalidomide in the urine within 24 hours.
Volume of Distribution
In healthy male subjects, the apparent volume of distribution was 75.8 7.3 L.
Clearance
The renal clearance of lenalidomide exceeds the glomerular filtration rate. In healthy male subjects, the oral clearance was 318 41 mL/min.
In vitro (14)C-lenalidomide binding to plasma proteins is approximately 30%.
NIH; DailyMed. Current Medication Information for Revlimid (Lenalidomide) Capsule (Revised: September 2014). Available from, as of February 23, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5fa97bf5-28a2-48f1-8955-f56012d296be
Administration of a single 25 mg dose of Revlimid with a high-fat meal in healthy subjects reduces the extent of absorption, with an approximate 20% decrease in AUC and 50% decrease in Cmax. In the trials where the efficacy and safety were established for Revlimid, the drug was administered without regard to food intake. Revlimid can be administered with or without food.
NIH; DailyMed. Current Medication Information for Revlimid (Lenalidomide) Capsule (Revised: September 2014). Available from, as of February 23, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5fa97bf5-28a2-48f1-8955-f56012d296be
Systemic exposure (AUC) of lenalidomide in multiple myeloma (MM) and myelodysplastic syndromes (MDS) patients with normal or mild renal function (CLcr = 60 mL/min) is approximately 60% higher as compared to young healthy male subjects.
NIH; DailyMed. Current Medication Information for Revlimid (Lenalidomide) Capsule (Revised: September 2014). Available from, as of February 23, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5fa97bf5-28a2-48f1-8955-f56012d296be
Lenalidomide is rapidly absorbed following oral administration. Following single and multiple doses of Revlimid in patients with multiple myeloma (MM) or myelodysplastic syndromes (MDS) the maximum plasma concentrations occurred between 0.5 and 6 hours post-dose. The single and multiple dose pharmacokinetic disposition of lenalidomide is linear with AUC and Cmax values increasing proportionally with dose. Multiple dosing at the recommended dose-regimen does not result in drug accumulation.
NIH; DailyMed. Current Medication Information for Revlimid (Lenalidomide) Capsule (Revised: September 2014). Available from, as of February 23, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5fa97bf5-28a2-48f1-8955-f56012d296be
For more Absorption, Distribution and Excretion (Complete) data for Lenalidomide (9 total), please visit the HSDB record page.
Lenalidomide is not subject to extensive hepatic metabolism involving CYP enzymes and metabolism contributes to a very minor extent to the clearance of lenalidomide in humans. Lenalidomide undergoes hydrolysis in human plasma to form 5-hydroxy-lenalidomide and N-acetyl-lenalidomide. Unchanged lenalidomide is the predominant circulating drug form, with metabolites accounting for less than five percent of the parent drug levels in the circulation.
Lenalidomide -undergoes limited metabolism. Unchanged lenalidomide is the predominant circulating component in humans. Two identified metabolites are hydroxy-lenalidomide and N-acetyl-lenalidomide; each constitutes less than 5% of parent levels in circulation.
NIH; DailyMed. Current Medication Information for Revlimid (Lenalidomide) Capsule (Revised: September 2014). Available from, as of February 23, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5fa97bf5-28a2-48f1-8955-f56012d296be
In healthy subjects, the mean half-life of lenalidomide is three hours in the clinically relevant dose range (550 mg). Half-life can range from three to five hours in patients with multiple myeloma, myelodysplastic syndromes, or mantle cell lymphoma.
The mean half-life of lenalidomide is 3 hours in healthy subjects and 3 to 5 hours in patients with multiple myeloma (MM), myelodysplastic syndromes (MDS) or mantle cell lymphoma (MCL).
NIH; DailyMed. Current Medication Information for Revlimid (Lenalidomide) Capsule (Revised: September 2014). Available from, as of February 23, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5fa97bf5-28a2-48f1-8955-f56012d296be
Lenalidomide is a drug with multiple mechanisms of action. Lenalidomide exerts immunomodulating effects by altering cytokine production, regulating T cell co-stimulation, and enhancing the NK cell-mediated cytotoxicity. Lenalidomide directly inhibits the cullin ring E3 ubiquitin ligase complex: upon binding to cereblon, a substrate adaptor of the complex, lenalidomide modulates substrate specificity of the complex to recruit substrate proteins of the ligase, including Ikaros (IKZF1), Aiolos (IKZF3), and CK1. These substrates are then tagged for ubiquitination and subsequent proteasomal degradation. IKZF1 and IKZF3 are B-cell transcription factors that are essential for B-cell differentiation and survival of malignant cells. IKZF3 also regulates the expression of interferon regulatory factor 4 (IRF4), which is a transcription factor that regulates the aberrant myeloma-specific gene. The immunomodulatory actions of lenalidomide can be partly explained by the degradation of IKZF3, since it is a repressor of the interleukin 2 gene (IL2): as lenalidomide decreases the level of IKZF3, the production of IL-2 increases, thereby increasing the proliferation of natural killer (NK), NKT cells, and CD4+ T cells. Lenalidomide inhibits the production of pro-inflammatory cytokines TNF-, IL-1, IL-6, and IL-12, while elevating the production of anti-inflammatory cytokine IL-10. Lenalidomide acts as a T-cell co-stimulatory molecule that promotes CD3 T-cell proliferation and increases the production of IL-2 and IFN- in T lymphocytes, which enhances NK cell cytotoxicity and ADCC. It inhibits the expression and function of T-regulatory cells, which are often overabundant in some hematological malignancies. Lenalidomide directly exerts antitumour effects by inhibiting the proliferation and inducing apoptosis of tumour cells. Lenalidomide triggers the activation of pro-apoptotic caspase-8, enhances tumour cell sensitivity to FAS-induced apoptosis, and downregulates NF-B, an anti-apoptotic protein. Independent of its immunomodulatory effects, lenalidomide mediates anti-angiogenic effects by inhibiting angiogenic growth factors released by tumour cells, such as vascular endothelial growth factor (VEGF), basic fibroblastic-growth factor (BFGF), and hepatocyte-growth factor. _In vitro_, lenalidomide inhibits cell adhesion molecules such as ICAM-1, LFA-1, 2 and 3 integrins, as well as gap-junction function, thereby preventing metastasis of malignant cells.
Multiple myeloma is a B-cell malignancy characterized by an excess of monotypic plasma cells in the bone marrow. The molecular mechanisms that are involved in disease progression depend on the interaction between the multiple myeloma cells and the bone microenvironment. Because these mechanisms have been well characterized, it is possible to develop regimens that are more specific to pathways involved in the pathogenesis of multiple myeloma than is typical for conventional chemotherapy in disease management. Thalidomide and immunomodulatory drugs (IMiDs) have now been shown to block several pathways important for disease progression in multiple myeloma. First established as agents with antiangiogenic properties, thalidomide and IMiDs inhibit the production of interleukin (IL)-6, which is a growth factor for the proliferation of myeloma cells. In addition, they activate apoptotic pathways through caspase 8-mediated cell death. At the mitochondrial level, they are responsible for c-jun terminal kinase (JNK)-dependent release of cytochrome-c and Smac into the cytosol of cells, where they regulate the activity of molecules that affect apoptosis. By activating T cells to produce IL-2, thalidomide and IMiDs alter natural killer (NK) cell numbers and function, thus augmenting the activity of NK-dependent cytotoxicity. Data delineating these events have been derived from experiments done in resistant and sensitive multiple myeloma cell lines. Although thalidomide and IMiDs demonstrate similar biologic activities, IMiDs are more potent than thalidomide and achieve responses at lower doses. Lenalidomide, a thalidomide derivative, has also been shown to have a different toxicity profile. Our understanding of the mechanism of action of these agents has provided a platform for exciting clinical trials evaluating combinations of thalidomide and lenalidomide with both conventional chemotherapy and newer targeted agents.
PMID:16344099 Anderson KC; Semin Hematol 42 (4 Suppl 4): S3-8 (2005)
Lenalidomide is an analogue of thalidomide with immunomodulatory, antiangiogenic, and antineoplastic properties. Lenalidomide inhibits proliferation and induces apoptosis of certain hematopoietic tumor cells including multiple myeloma, mantle cell lymphoma, and del (5q) myelodysplastic syndromes in vitro. Lenalidomide causes a delay in tumor growth in some in vivo nonclinical hematopoietic tumor models including multiple myeloma. Immunomodulatory properties of lenalidomide include activation of T cells and natural killer (NK) cells, increased numbers of NKT cells, and inhibition of pro-inflammatory cytokines (e.g., TNF-alpha and IL-6) by monocytes. In multiple myeloma cells, the combination of lenalidomide and dexamethasone synergizes the inhibition of cell proliferation and the induction of apoptosis.
NIH; DailyMed. Current Medication Information for Revlimid (Lenalidomide) Capsule (Revised: September 2014). Available from, as of February 23, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5fa97bf5-28a2-48f1-8955-f56012d296be
Although several mechanisms have been proposed to explain the activity of thalidomide, lenalidomide and pomalidomide in multiple myeloma (MM), including demonstrable anti-angiogenic, anti-proliferative and immunomodulatory effects, the precise cellular targets and molecular mechanisms have only recently become clear. A landmark study recently identified cereblon (CRBN) as a primary target of thalidomide teratogenicity. Subsequently it was demonstrated that CRBN is also required for the anti-myeloma activity of thalidomide and related drugs, the so-called immune-modulatory drugs (IMiDs). Low CRBN expression was found to correlate with drug resistance in MM cell lines and primary MM cells. One of the downstream targets of CRBN identified is interferon regulatory factor 4 (IRF4), which is critical for myeloma cell survival and is down-regulated by IMiD treatment. CRBN is also implicated in several effects of IMiDs, such as down-regulation of tumor necrosis factor-alpha (TNF-a) and T cell immunomodulatory activity, demonstrating that the pleotropic actions of the IMiDs are initiated by binding to CRBN. Future dissection of CRBN downstream signaling will help to delineate the underlying mechanisms for IMiD action and eventually lead to development of new drugs with more specific anti-myeloma activities. It may also provide a biomarker to predict IMiD response and resistance.
PMID:22966948 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3931443 Zhu YX et al; Leuk Lymphoma 54 (4): 683-7 (2013)
Immunomodulatory drugs lenalidomide and pomalidomide are synthetic compounds derived by modifying the chemical structure of thalidomide to improve its potency and reduce its side effects. Lenalidomide is a 4-amino-glutamyl analogue of thalidomide that lacks the neurologic side effects of sedation and neuropathy and has emerged as a drug with activity against various hematological and solid malignancies. It is approved by FDA for clinical use in myelodysplastic syndromes with deletion of chromosome 5q and multiple myeloma. Lenalidomide has been shown to be an immunomodulator, affecting both cellular and humoral limbs of the immune system. It has also been shown to have anti-angiogenic properties. Newer studies demonstrate its effects on signal transduction that can partly explain its selective efficacy in subsets of MDS. Even though the exact molecular targets of lenalidomide are not well known, its activity across a spectrum of neoplastic conditions highlights the possibility of multiple target sites of action.
PMID:19674465 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2736171 Kotla V et al; J Hematol Oncol. 2009 Aug 12;2:36. doi: 10.1186/1756-8722-2-36
For more Mechanism of Action (Complete) data for Lenalidomide (7 total), please visit the HSDB record page.