


1. Lasiocarpine Hydrochloride, (1s-(1alpha(z),7(2s*,3r*),7aalpha))-isomer
2. Lasiocarpine Sulfate, (1s-(1alpha(z),7(2s*,3r*),7aalpha))-isomer
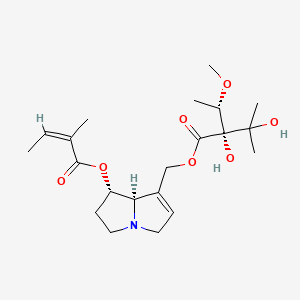
1. 7-angelyleuropine
2. (-)-lasiocarpine
3. 303-34-4
4. Europine 7-angelate
5. Nsc-30625
6. Nci-c01478
7. S770100q96
8. Rcra Waste Number U143
9. Mls002639334
10. Heliotridine Ester With Lasiocarpum And Angelic Acid
11. Ccris 355
12. Nsc30625
13. C21h33no7
14. Hsdb 6062
15. Unii-s770100q96
16. Nsc 30625
17. Heliotridine Ester With Lasiocarpum & Angelic Acid
18. Ai3-51770
19. Lasiocarpine [mi]
20. Lasiocarpine, Hplc Grade
21. Rcra Waste No. U143
22. Lasiocarpine [hsdb]
23. Lasiocarpine [iarc]
24. Schembl168932
25. Chembl1999554
26. Mfcd31631257
27. Zinc61997632
28. Heliosupine Methyl Ether [mi]
29. Akos030501885
30. (z)-2-methylcrotonic Acid, 2,3-dihydroxy-2-(1-methoxyethyl)-3-methylbutyrate (ester)
31. (7alpha-angelyloxy-5,6,7,8alpha-tetrahydro-3h-pyrrolizin-1-yl)methyl-2,3-dihydroxy-2-(1'-methoxyethyl)-3-methylbutyrate
32. 2,3,5,7alphabeta-tetrahydro-1-hydroxy-1h-pyrrolizine-7-methanol-1-angelate-7-(2,3-dihydroxy-2(1-methoxyethyl))-3-methyl-butyrate
33. 2-butenoic Acid, 2-methyl-, 7-((2,3-dihydroxy-2-(1-methoxyethyl)-3-methyl-1-oxobutoxy)methyl)-2,3,5,7a-tetrahydro-1h-pyrrolizin-1-yl Ester, (1s-(1alpha(z),7(2s*,3r*),7aalpha))-
34. Wln: T55 An Cutj D1ovxqxq1 & 1 & Y1 & O1 Fovy1 & U2
35. (1s,7ar)-7-((((r)-2,3-dihydroxy-2-((s)-1-methoxyethyl)-3-methylbutanoyl)oxy)methyl)-2,3,5,7a-tetrahydro-1h-pyrrolizin-1-yl (z)-2-methylbut-2-enoate
36. 2-butenoic Acid, 2-methy-,(1s,7ar)-7-(((2r)-2,3-dihydroxy-2-((1s)-1-methoxyethyl)-3-methyl-1-oxobutoxy)methyl)-2,3,5,7a-tetrahydro-1h-ester, (2z)-
37. 2-butenoic Acid, 2-methyl-, 7-((2,3-dihydroxy-2-(1-methoxyethyl)-3-methyl-1-oxobutoxy)methyl)-2,3,5,7a-tetrahydro-1h-pyrrolizin-1-yl Ester, (1s-(1.alpha.(z),7(s*(r*)),7a.alpha.))-
38. 2-butenoic Acid, 2-methyl-, 7-((2,3-dihydroxy-2-(1-methoxyethyl)-3-methyl-1-oxobutoxy)methyl)-2,3,5,7a-tetrahydro-1h-pyrrolizin-1-yl Ester, (1s-(1alpha(z),7(2s*,3r*),7a Alpha))-
39. 2-butenoic Acid, 7-[[2,3-dihydroxy-2-(1-methoxyethyl)-3-methyl-1-oxobutoxy]methyl]-2,3,5,7a-tetrahydro-1h-pyrrolizin-1-yl Ester, [1s-[1.alpha.(z),7(2s*,3r*),7a.alpha.]]-
| Molecular Weight | 411.5 g/mol |
|---|---|
| Molecular Formula | C21H33NO7 |
| XLogP3 | 0.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 10 |
| Exact Mass | 411.22570239 g/mol |
| Monoisotopic Mass | 411.22570239 g/mol |
| Topological Polar Surface Area | 106 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 699 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
LASROCARPINE (RANDOMLY LABELLED, 44% IN THE AMINO ALCOHOL) WAS ADMIN IP TO RATS (TOTAL DOSE, 5 MG) DISTRIBUTION OF LABEL AFTER 4 HR WAS AS FOLLOWS: CARCASS, 6.4%; INTESTINES, 8.6%; TESTES, 0.1%; LUNG, 0.05%; KIDNEY, 0.26%; HEART, 0.05%; SPLEEN, 0.01%; BRAIN, 0.03%; URINE, 27.2%; LIVER, 2.8%, EXPIRED CO2, 9.3% FRACTIONATION OF LIVER RESULTED IN 1.73% IN A TRICHLOROACETIC ACID EXTRACT, 0.6% IN PROTEIN, 0.48% IN LIPID & 0.005% IN NUCLEIC ACIDS.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V10 285 (1976)
STUDIES WITH LASIOCARPINE HAVE CONFIRMED THE FORMATION OF PYRROLIC METABOLITES BY THE MIXED-FUNCTION OXIDASE SYSTEM OF THE MICROSOMAL FRACTION OF RAT LIVER. DEHYDROHELIOTRIDINE HAS BEEN ISOLATED & IDENTIFIED AS A PRODUCT OF MICROSOMAL OXIDATION OF LASIOCARPINE.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V10 285 (1976)
IN URINE ... OBTAINED 16 HR AFTER INJECTION ... TO RATS ... UNCHANGED LASIOCARPINE (1-1.5% OF DOSE), HELIOTRIDINE (1.5-3%), HELIOTRIDINE N-OXIDE (6%) & TRACES OF BASES WITH CHROMATOGRAPHIC PROPERTIES OF EUROPINE & 7-ANGELYHELIOTRIDINE (EXPECTED PRODUCTS OF PARTIAL HYDROLYSIS). ... METABOLITES IN 24-HR URINE SAMPLE ... 8.5% OF ADMIN LASIOCARPINE.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V10 286 (1976)
HUMAN EMBRYONIC LIVER SLICES CONVERTED THE PYRROLIZIDINE ALKALOID LASIOCARPINE INTO PYRROLES, AS INDICATED BY A POS EHRLICH COLOR REACTION, WHEREAS LUNG TISSUE DID NOT.
ARMSTRONG SJ, ZUCKERMAN AJ; NATURE (LONDON) 228 (5271): 569 (1970)
Death may be due to actual liver damage or to the upsetting of the copper storage mechanism which leads to a build-up of copper in the organ, resulting in the acute hemolytic crisis associated with chronic copper poisoning.
Humphreys, D.J. Veterinary Toxicology. 3rd ed. London, England: Bailliere Tindell, 1988., p. 236
