


1. Gw 282974x
2. Gw 572016
3. Gw-282974x
4. Gw-572016
5. Gw282974x
6. Gw572016
7. Lapatinib
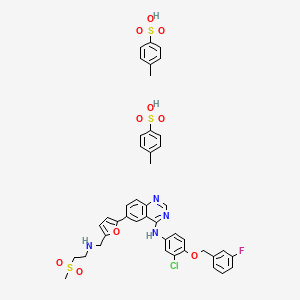
8. N-(3-chloro-4-(((3-fluorobenzyl)oxy)phenyl)-6-(5-(((2-methylsulfonyl)ethyl)amino)methyl) -2-furyl)-4-quinazolinamine
9. Tykerb
1. 388082-77-7
2. Tykerb
3. Tykerb Ditosylate
4. Lapatinib (gw-572016) Ditosylate
5. Tyverb
6. Lapatinib (ditosylate)
7. N-(3-chloro-4-((3-fluorobenzyl)oxy)phenyl)-6-(5-(((2-(methylsulfonyl)ethyl)amino)methyl)furan-2-yl)quinazolin-4-amine Bis(4-methylbenzenesulfonate)
8. 388082-78-8
9. Lapatinib Ditosylate Anhydrous
10. 4wk72k94mc
11. 388082-77-7 (ditosylate)
12. Bis(4-methylbenzene-1-sulfonic Acid); N-{3-chloro-4-[(3-fluorophenyl)methoxy]phenyl}-6-(5-{[(2-methanesulfonylethyl)amino]methyl}furan-2-yl)quinazolin-4-amine
13. Gw-572016
14. 4-quinazolinamine, N-(3-chloro-4-((3-fluorophenyl)methoxy)phenyl)-6-(5-(((2-(methylsulfonyl)ethyl)amino)methyl)-2-furanyl)-, Bis(4-methylbenzenesulfonate)
15. N-(3-chloro-4-((3-fluorobenzyl)oxy)phenyl)-6-(5-(((2-(methylsulfonyl)ethyl)amino)methyl)furan-2-yl)quinazolin-4-amine Ditosylate
16. Sr-05000001472
17. N-[3-chloro-4-[(3-fluorophenyl)methoxy]phenyl]-6-[5-[(2-methylsulfonylethylamino)methyl]furan-2-yl]quinazolin-4-amine;4-methylbenzenesulfonic Acid
18. S1028
19. Unii-4wk72k94mc
20. Gw 572016f
21. Gw-572016 Ditosylate
22. Lapatinib Ditosylate,tykerb
23. Schembl93590
24. Quinazolin-4-amine Ditosylate
25. Chembl1201183
26. Dtxsid60959606
27. Hms3265i13
28. Hms3265i14
29. Hms3265j13
30. Hms3265j14
31. Hms3654e07
32. Hy-50898a
33. Mfcd09264195
34. Akos015888607
35. Ac-5247
36. Ccg-264661
37. Cs-0831
38. Gs-3661
39. Db-119273
40. Ft-0670728
41. Lapatinib Ditoluenesulfonate Anhydrous
42. Sw199101-4
43. Ec-000.2339
44. Lapatinib Ditosylate Anhydrous [who-dd]
45. Gw 572016 Ditosylate;gw-572016 Ditosylate
46. Lapatinib Ditoluenesulfonate Anhydrous [mi]
47. Sr-05000001472-2
48. Sr-05000001472-5
49. Q27260601
50. Z1692482592
51. 4-methylbenzene-1-sulfonic Acid--n-{3-chloro-4-[(3-fluorophenyl)methoxy]phenyl}-6-[5-({[2-(methanesulfonyl)ethyl]amino}methyl)furan-2-yl]quinazolin-4-amine (2/1)
52. 4-quinazolinamine, N-(3-chloro-4-((3-fluorophenyl)methoxy)phenyl)-6-(5-(((2-(methylsulfonyl)ethyl)amino)methyl)-2-furanyl)-, 4-methylbenzenesulfonate (1:2)
53. N-(3-chloro-4-((3-fluorobenzyl)oxy)phenyl)-6-(5-(((2-(methylsulfonyl)ethyl)amino)methyl)furan-2-yl)quinazolin-4-amine Bis(4-methylbenzenesulfona
54. N-(3-chloro-4-(3-fluorobenzyloxy)phenyl)-6-(5-((2-(methylsulfonyl) Ethylamino)methyl)furan-2-yl)quinazolin-4-amine Ditosylate
55. N-(3-chloro-4-(3-fluorobenzyloxy)phenyl)-6-(5-((2-(methylsulfonyl)ethylamino)methyl)furan-2-yl)quinazolin-4-amine Ditosylate
56. N-[3-chloro-4-[(3-fluorophenyl)methoxy]phenyl]-6-[5-[(2-methylsulfonylethylamino)methyl]-2-furyl]
| Molecular Weight | 925.5 g/mol |
|---|---|
| Molecular Formula | C43H42ClFN4O10S3 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 15 |
| Rotatable Bond Count | 13 |
| Exact Mass | 924.1735629 g/mol |
| Monoisotopic Mass | 924.1735629 g/mol |
| Topological Polar Surface Area | 240 Ų |
| Heavy Atom Count | 62 |
| Formal Charge | 0 |
| Complexity | 1100 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
| 1 of 2 | |
|---|---|
| Drug Name | Tykerb |
| PubMed Health | Lapatinib (By mouth) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | Lapatinib is a small molecule and a member of the 4-anilinoquinazoline class of kinase inhibitors. It is present as the monohydrate of the ditosylate salt, with chemical name N-(3-chloro-4-{[(3-fluorophenyl)methyl]oxy}phenyl)-6-[5-({[2-(methylsulfony... |
| Active Ingredient | Lapatinib ditosylate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 250mg base |
| Market Status | Prescription |
| Company | Smithkline Beecham |
| 2 of 2 | |
|---|---|
| Drug Name | Tykerb |
| PubMed Health | Lapatinib (By mouth) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | Lapatinib is a small molecule and a member of the 4-anilinoquinazoline class of kinase inhibitors. It is present as the monohydrate of the ditosylate salt, with chemical name N-(3-chloro-4-{[(3-fluorophenyl)methyl]oxy}phenyl)-6-[5-({[2-(methylsulfony... |
| Active Ingredient | Lapatinib ditosylate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 250mg base |
| Market Status | Prescription |
| Company | Smithkline Beecham |
Tyverb is indicated for the treatment of patients with breast cancer , whose tumours overexpress HER2 (ErbB2):
- in combination with capecitabine for patients with advanced or metastatic disease with progression following prior therapy, which must have included anthracyclines and taxanes and therapy with trastuzumab in the metastatic setting;
- in combination with trastuzumab for patients with hormone-receptor-negative metastatic disease that has progressed on prior trastuzumab therapy or therapies in combination with chemotherapy;
- in combination with an aromatase inhibitor for post-menopausal women with hormone-receptor-positive metastatic disease, not currently intended for chemotherapy. The patients in the registration study had not previously been treated with trastuzumab or an aromatase inhibitor. No data are available on the efficacy of this combination relative to trastuzumab in combination with an aromatase inhibitor in this patient population.
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Protein Kinase Inhibitors
Agents that inhibit PROTEIN KINASES. (See all compounds classified as Protein Kinase Inhibitors.)
L01EH01
