


1. Amuno
2. Indocid
3. Indocin
4. Indomet 140
5. Indometacin
6. Indomethacin
7. Indomethacin Hydrochloride
8. Metindol
9. Osmosin
1. 7681-54-1
2. Osmosin
3. Sodium Indomethacin
4. Indomethacin (sodium)
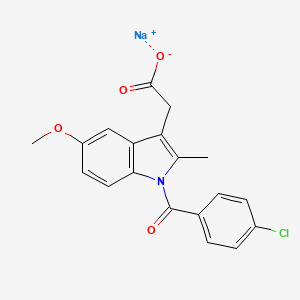
5. Sodium 2-(1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1h-indol-3-yl)acetate
6. Indometacin (sodium)
7. Indocin I.v.
8. Indomethacin Sodium Anhydrous
9. 1h-indole-3-acetic Acid, 1-(4-chlorobenzoyl)-5-methoxy-2-methyl-, Sodium Salt
10. 1c9d998830
11. Sodium;2-[1-(4-chlorobenzoyl)-5-methoxy-2-methylindol-3-yl]acetate
12. Sodium 1-(p-chlorobenzoyl)-5-methoxy-2-methylindole-3-acetate
13. Einecs 231-670-4
14. Sodium 1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1h-indole-3-acetate
15. Unii-1c9d998830
16. Indomethacinsodium
17. Indole-3-acetic Acid, 1-(p-chlorobenzoyl)-5-methoxy-2-methyl-, Sodium Salt
18. Schembl3772654
19. Dtxsid20227631
20. Akos016014921
21. Ac-13625
22. Hy-15034
23. Cs-0003710
24. Q27252232
25. Sodium,2-[1-(4-chlorobenzoyl)-5-methoxy-2-methylindol-3-yl]acetate
26. 1h-indole-3-acetic Acid, 1-(4-chlorobenzoyl)-5-methoxy-2-methyl-, Sodium Salt (1:1)
| Molecular Weight | 379.8 g/mol |
|---|---|
| Molecular Formula | C19H15ClNNaO4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 4 |
| Exact Mass | 379.0587299 g/mol |
| Monoisotopic Mass | 379.0587299 g/mol |
| Topological Polar Surface Area | 71.4 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 512 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Indomethacin sodium |
| Active Ingredient | Indomethacin sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 1mg base/vial |
| Market Status | Prescription |
| Company | Eurohlth Intl |
| 2 of 2 | |
|---|---|
| Drug Name | Indomethacin sodium |
| Active Ingredient | Indomethacin sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 1mg base/vial |
| Market Status | Prescription |
| Company | Eurohlth Intl |
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
Cardiovascular Agents
Agents that affect the rate or intensity of cardiac contraction, blood vessel diameter, or blood volume. (See all compounds classified as Cardiovascular Agents.)
Cyclooxygenase Inhibitors
Compounds or agents that combine with cyclooxygenase (PROSTAGLANDIN-ENDOPEROXIDE SYNTHASES) and thereby prevent its substrate-enzyme combination with arachidonic acid and the formation of eicosanoids, prostaglandins, and thromboxanes. (See all compounds classified as Cyclooxygenase Inhibitors.)
Gout Suppressants
Agents that increase uric acid excretion by the kidney (URICOSURIC AGENTS), decrease uric acid production (antihyperuricemics), or alleviate the pain and inflammation of acute attacks of gout. (See all compounds classified as Gout Suppressants.)
Tocolytic Agents
Drugs that prevent preterm labor and immature birth by suppressing uterine contractions (TOCOLYSIS). Agents used to delay premature uterine activity include magnesium sulfate, beta-mimetics, oxytocin antagonists, calcium channel inhibitors, and adrenergic beta-receptor agonists. The use of intravenous alcohol as a tocolytic is now obsolete. (See all compounds classified as Tocolytic Agents.)
