


1. D-arg(hyp(3)-thi(5)-d-tic(7)-oic(8))bk
2. D-arg(hyp(3)-thi(5)-l-tic(7)-oic(8))bk
3. Firazyr
4. Hoe 140
5. Hoe-140
6. Hoe140
7. Hoechst 140
8. Hoechst-140
9. Icatibant
10. Je 049
11. Je-049
12. Win 65365
13. Win-65365
1. Icatibant Acetate [usan]
2. Icatibant (acetate)
3. Firazyr
4. 325o8467xk
5. 138614-30-9
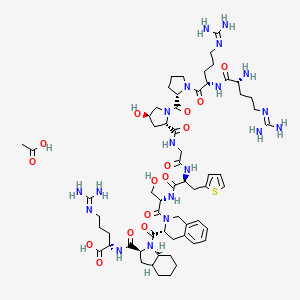
6. (r)-arginyl-(s)-arginyl-(s)-prolyl-(2s,4r)-(4-hydroxyprolyl)glycyl-(s)-(3-(2-thienyl)alanyl)-(s)-seryl-(r)-((1,2,3,4-tetrahydro-3-isoquinolyl)carbonyl)-(2s,3as,7as)-((hexahydro-2-indolinyl)carbonyl)-(s)-arginine Acetate (salt)
7. Hoe 140
8. Unii-325o8467xk
9. Icatibant Acetate [mi]
10. Icatibant Acetate [jan]
11. Chembl2028852
12. Icatibant Acetate [mart.]
13. Icatibant Acetate [who-dd]
14. Je-049
15. Icatibant Acetate [orange Book]
16. Hy-108896
17. Cs-0031296
18. Q27256146
19. L-arginine, D-arginyl-l-arginyl-l-prolyl-(4r)-4-hydroxy-l-prolylglycyl-3-(2-thienyl)-l-alanyl-l-seryl-(3r)-1,2,3,4-tetrahydro-3-isoquinolinecarbonyl-(2s,3as,7as)-octahydro-1h-indole-2-carbonyl-, Acetate
20. L-arginine, D-arginyl-l-arginyl-l-prolyl-trans-4-hydroxy-l-prolylglycyl-3-(2-thienyl)-l-alanyl-l-seryl-d-1,2,3,4-tetrahydro-3-isoquinolinecarbonyl-l-(2.alpha.,3a.beta.,7a.beta.)-octahydro-1h-indole-2-carbonyl-, Acetate (salt)
21. L-arginine, D-arginyl-l-arginyl-l-prolyl-trans-4-hydroxy-l-prolylglycyl-3-(2-thienyl)-l-alanyl-l-seryl-d-1,2,3,4-tetrahydro-3-isoquinolinecarbonyl-l-(2alpha,3abeta,7abeta)-octahydro-1h-indole-2-carbonyl-, Acetate (salt)
| Molecular Weight | 1364.6 g/mol |
|---|---|
| Molecular Formula | C61H93N19O15S |
| Hydrogen Bond Donor Count | 16 |
| Hydrogen Bond Acceptor Count | 20 |
| Rotatable Bond Count | 30 |
| Exact Mass | 1363.68192451 g/mol |
| Monoisotopic Mass | 1363.68192451 g/mol |
| Topological Polar Surface Area | 589 Ų |
| Heavy Atom Count | 96 |
| Formal Charge | 0 |
| Complexity | 2750 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 12 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Firazyr is indicated for symptomatic treatment of acute attacks of hereditary angioedema (HAE) in adults (with C1-esterase-inhibitor deficiency).
Treatment of hereditary angioedema
Icatibant Accord is indicated for symptomatic treatment of acute attacks of hereditary angioedema (HAE) in adults, adolescents and children aged 2 years and older, with C1 esterase inhibitor deficiency.
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
Bradykinin B2 Receptor Antagonists
Compounds and drugs that inhibit ligand binding or cellular signaling by BRADYKININ B2 RECEPTORS. (See all compounds classified as Bradykinin B2 Receptor Antagonists.)
B06AC02
B06AC02
