

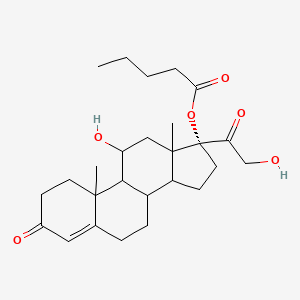

1. Ncgc00018263-02
2. Chembl67128
3. Chebi:181452
4. Akos015917554
| Molecular Weight | 446.6 g/mol |
|---|---|
| Molecular Formula | C26H38O6 |
| XLogP3 | 3.8 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 7 |
| Exact Mass | 446.26683893 g/mol |
| Monoisotopic Mass | 446.26683893 g/mol |
| Topological Polar Surface Area | 101 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 832 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 6 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Hydrocortisone valerate |
| PubMed Health | Hydrocortisone Valerate (Topical application route) |
| Drug Classes | Corticosteroid, Intermediate, Hydrocortisone |
| Active Ingredient | Hydrocortisone valerate |
| Dosage Form | Ointment; Cream |
| Route | Topical |
| Strength | 0.2% |
| Market Status | Prescription |
| Company | Taro; Perrigo New York |
| 2 of 2 | |
|---|---|
| Drug Name | Hydrocortisone valerate |
| PubMed Health | Hydrocortisone Valerate (Topical application route) |
| Drug Classes | Corticosteroid, Intermediate, Hydrocortisone |
| Active Ingredient | Hydrocortisone valerate |
| Dosage Form | Ointment; Cream |
| Route | Topical |
| Strength | 0.2% |
| Market Status | Prescription |
| Company | Taro; Perrigo New York |

