

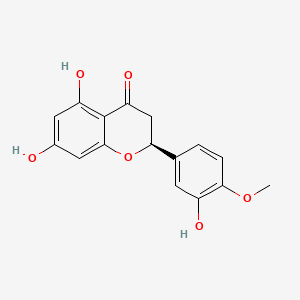

1. (-)-hesperetin
2. 3',5,7-trihydroxy-4'-methoxyflavanone
3. Hesperitin
1. 520-33-2
2. Hesperitin
3. 3',5,7-trihydroxy-4'-methoxyflavanone
4. (-)-hesperetin
5. (s)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)chroman-4-one
6. Yso2
7. 5,7,3'-trihydroxy-4'-methoxyflavanone
8. Cyanidanon 4'-methyl Ether 1626
9. 41001-90-5
10. Nsc 57654
11. Prestwick_908
12. (2s)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-2,3-dihydrochromen-4-one
13. (-)-(s)-hesperetin
14. Nsc-57654
15. 4h-1-benzopyran-4-one, 2,3-dihydro-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-, (s)-
16. Eriodictyol 4'-monomethyl Ether
17. Chebi:28230
18. Q9q3d557f1
19. (2s)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-2,3-dihydro-4h-chromen-4-one
20. (2s)-hesperetin
21. (2s)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)chroman-4-one
22. (s)-2,3-dihydro-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4h-1-benzopyran-4-one
23. (+-)-hesperetin
24. Mfcd00075646
25. (+/-)-hesperetin
26. (2s)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-2,3-dihydro-4h-1-benzopyran-4-one
27. Einecs 208-290-2
28. Hesperitine
29. Unii-q9q3d557f1
30. 4h-1-benzopyran-4-one, 2,3-dihydro-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-, (2s)-
31. (2s)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-2,3-dihydro-4h-1-benzopyran-4-one (hesperetin)
32. 6jp
33. Tnp00238
34. (s)-2,3-dihydro-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4-benzopyrone
35. 4'-methoxy-3',5,7-trihydroxyflavanone
36. Spectrum_000181
37. Hesperetin [mi]
38. Prestwick0_000124
39. Prestwick1_000124
40. Prestwick2_000124
41. Prestwick3_000124
42. Spectrum2_001793
43. Spectrum3_001104
44. Spectrum4_001935
45. Spectrum5_000683
46. Hesperetin [fhfi]
47. Hesperetin [inci]
48. 5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4-chromanone
49. Oprea1_828704
50. Schembl39833
51. Bspbio_000168
52. Bspbio_002808
53. Kbiogr_002311
54. Kbioss_000661
55. Spectrum310012
56. Flavanone, 3',5,7-trihydroxy-4'-methoxy- (van)
57. Mls002154205
58. Bidd:er0512
59. Divk1c_001039
60. Spbio_001745
61. Spbio_002107
62. Bpbio1_000186
63. Chembl399121
64. Dtxsid4022319
65. Fema No. 4313
66. Bcbcmap01_000087
67. Bdbm23418
68. Gtpl10953
69. Hms503o19
70. Kbio1_001039
71. Kbio2_000661
72. Kbio2_003229
73. Kbio2_005797
74. Kbio3_002028
75. Zinc39092
76. Ninds_001039
77. Hms1568i10
78. Hms2095i10
79. Hms2230m09
80. Hms3649h22
81. Hms3884n11
82. Np-13
83. (2s)-5,7-dihydroxy-2-(3-hydroxy-4-methoxy-phenyl)chroman-4-one
84. Bcp28273
85. Hy-n0168
86. Bbl104011
87. Ccg-38441
88. Lmpk12140003
89. S2308
90. Stl557824
91. Akos016339567
92. Ac-7970
93. Db01094
94. Ks-5307
95. Sdccgmls-0066605.p001
96. Idi1_001039
97. Smp1_000148
98. Ncgc00016482-01
99. Ncgc00016482-02
100. Ncgc00016482-03
101. Ncgc00016482-04
102. Ncgc00142415-01
103. Ncgc00142415-02
104. 5,7, 3'-trihydroxy-4'-methoxyflavanone
105. Cas-520-33-2
106. Smr001233491
107. Flavanone, 3',5,7-trihydroxy-4'-methoxy-
108. H0721
109. Hesperitin; Hesperin; Yso2; Prestwick_908
110. N1856
111. Sw197026-2
112. C01709
113. H10029
114. A828900
115. Discontinued. Please See H289480 Or H289501
116. Q411310
117. Sr-01000946723
118. Sr-01000946723-1
119. Brd-k30553453-001-05-8
120. Brd-k30553453-001-08-2
121. Flavanone, 3',5, 7-trihydroxy-4'-methoxy- (van)
122. Flavanone, 3',5,7-trihydroxy-4'-methoxy- (van) (8ci)
123. (s)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)chroman-4-one;hesperetin
124. (2s)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-3,4-dihydro-2h-1-benzopyran-4-one
125. 2,3-dihydro-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4h-1-benzopyran-4-one
126. 4h-1-benzopyran-4-one,2,3-dihydro-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-, (2s)-
| Molecular Weight | 302.28 g/mol |
|---|---|
| Molecular Formula | C16H14O6 |
| XLogP3 | 2.4 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | 302.07903816 g/mol |
| Monoisotopic Mass | 302.07903816 g/mol |
| Topological Polar Surface Area | 96.2 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 413 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For lowering cholesterol and, possibly, otherwise favorably affecting lipids. In vitro research also suggests the possibility that hesperetin might have some anticancer effects and that it might have some anti-aromatase activity, as well as activity again.
Hesperetin is a cholesterol lowering flavanoid found in a number of citrus juices. It appears to reduce cholesteryl ester mass and inhibit apoB secretion by up to 80%. Hesperetin may have antioxidant, anti-inflammatory, anti-allergic, hypolipidemic, vasoprotective and anticarcinogenic actions.
Hesperetin has known human metabolites that include Hesperetin 3p-O-glucuronide and Hesperetin 7-O-glucuronide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Hesperetin reduces or inhibits the activity of acyl-coenzyme A:cholesterol acyltransferase genes (ACAT1 and ACAT2) and it reduces microsomal triglyceride transfer protein (MTP) activity. Hesperetin also seems to upregulate the LDL receptor. This leads to the reduced assembly and secretion of apoB-containing lipoproteins and enhanced reuptake of those lipoproteins, thereby lowering cholesterol levels.
