


1. (+)-halofuginone
2. (+-)-trans-7-bromo-6-chloro-3-(3-(3-hydroxy-2-piperidyl)-acetonyl)-4(3h)-quinazolinone
3. (+-)-trans-7-bromo-6-chloro-3-(3-(3-hydroxy-2-piperidyl)-acetonyl)-4(3h)-quinazolinone Monohydrobromide
4. (-)-halofuginone
5. (-)-halofuginone Hydrobromide
6. 4(3h)-quinazolinone, 7-bromo-6-chloro-3-(3-((2r,3s)-3-hydroxy-2-piperidinyl)-2-oxopropyl)-, Hydrochloride (1:1), Rel-
7. 4(3h)-quinazolinone, 7-bromo-6-chloro-3-(3-((2s,3r)-3-hydroxy-2-piperidinyl)-2-oxopropyl)-, Hydrobromide (1:1)
8. 4(3h)-quinazolinone, 7-bromo-6-chloro-3-(3-(3-hydroxy-2-piperidinyl)-2-oxopropyl)-, Hydrobromide, Trans-(+-)-
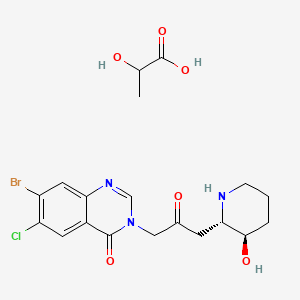
9. 4(3h)-quinazolinone, 7-bromo-6-chloro-3-(3-(3-hydroxy-2-piperidinyl)-2-oxopropyl)-, Trans-, Mono(2-hydroxypropanoate)(salt)
10. 6-chloro-7-bromo-(+)-febrifugine
11. 6-chloro-7-bromofebrifugine
12. 7-bromo-6-chloro-3,3-(3-hydroxy-2-piperidyl)acetonyl-4(3h)-quinazolinone-hydrolactate
13. 7-bromo-6-chloro-3-(3-((2r,3s)-3-hydroxy-2-piperidinyl)-2-oxopropyl)-4(3h)-quinazolinone
14. 7-bromo-6-chloro-3-(3-((2s,3r)-3-hydroxy-2-piperidinyl)-2-oxopropyl)-4(3h)-quinazolinone
15. 7-bromo-6-chloro-3-(3-((2s,3r)-3-hydroxy-2-piperidyl)-2-oxo-propyl)quinazolin-4-one Hydrobromide
16. 7-bromo-6-chloro-3-(3-(3-hydroxy-2-piperidinyl)-2-oxopropyl)-4(3h)-quinazolinone
17. 7-bromo-6-chlorofebrifugine
18. Cebegine
19. Chloro-bromofebrifugine
20. Chlorobromofebrifugine
21. Halagon
22. Halocur
23. Halofuginon
24. Halofuginone
25. Halofuginone Hbr
26. Halofuginone Hcl
27. Halofuginone Hydrobromide
28. Halofuginone Hydrobromide, (-)-
29. Halofuginone Hydrochloride
30. Halofuginone Monohydrobromide
31. Halofuginone Monohydrochloride
32. Halofuginone, (+)-
33. Halofuginone, (-)-
34. Halofunginone
35. Ru 19110
36. Ru-19110
37. Ru19110
38. Stenorol
39. Trans-7-bromo-6-chloro-3-(3-(3-hydroxy-2-piperidyl)-acetonyl)-4(3h)-quinazolinone
1. 82186-71-8
2. Schembl2562822
3. Chembl1162014
4. Ac-30636
5. 7-bromo-6-chloro-3-(3-((2s,3r)-3-hydroxypiperidin-2-yl)-2-oxopropyl)quinazolin-4(3h)-one 2-hydroxypropanoate
6. 7-bromo-6-chloro-3-[3-[(2s,3r)-3-hydroxypiperidin-2-yl]-2-oxopropyl]quinazolin-4-one;2-hydroxypropanoic Acid
| Molecular Weight | 504.8 g/mol |
|---|---|
| Molecular Formula | C19H23BrClN3O6 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 5 |
| Exact Mass | 503.04588 g/mol |
| Monoisotopic Mass | 503.04588 g/mol |
| Topological Polar Surface Area | 140 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 592 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
In newborn calves:
- Prevention of diarrhoea due to diagnosed Cryptosporidium parvum infection, in farms with history of cryptosporidiosis. Administration should start in the first 24 to 48 hours of age.
- Reduction of diarrhoea due to diagnosed Cryptosporidium parvum infection. Administration should start within 24 hours after the onset of diarrhoea. In both cases, the reduction of oocysts excretion has been demonstrated.
Angiogenesis Inhibitors
Agents and endogenous substances that antagonize or inhibit the development of new blood vessels. (See all compounds classified as Angiogenesis Inhibitors.)
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Coccidiostats
Agents useful in the treatment or prevention of COCCIDIOSIS in man or animals. (See all compounds classified as Coccidiostats.)
Protein Synthesis Inhibitors
Compounds which inhibit the synthesis of proteins. They are usually ANTI-BACTERIAL AGENTS or toxins. Mechanism of the action of inhibition includes the interruption of peptide-chain elongation, the blocking the A site of ribosomes, the misreading of the genetic code or the prevention of the attachment of oligosaccharide side chains to glycoproteins. (See all compounds classified as Protein Synthesis Inhibitors.)
QP51AX08
