


1. Glycomul S
2. Sorbitan Stearate
3. Sorgen 50
4. 1338-41-6
5. Sorbitan Monosterate
6. Schembl285527
7. Sorbitan Monostearate Kosher P
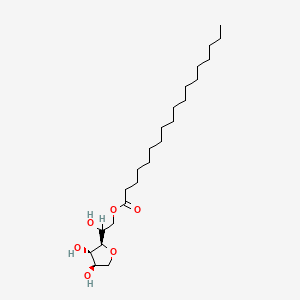
8. Octadecanoic Acid [2-[(2r,3s,4r)-3,4-dihydroxy-2-tetrahydrofuranyl]-2-hydroxyethyl] Ester
| Molecular Weight | 430.6 g/mol |
|---|---|
| Molecular Formula | C24H46O6 |
| XLogP3 | 6.9 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 20 |
| Exact Mass | 430.32943918 g/mol |
| Monoisotopic Mass | 430.32943918 g/mol |
| Topological Polar Surface Area | 96.2 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 417 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
The formulation and characterization of a novel anhydrous organogel formulated as a potential delivery vehicle from a solution of 2 nonionic surfactants, sorbitan monostearate (Span 60) and polysorbate 20 (polyoxyethylene sorbitan monolaurate; Tween 20), in hexadecane at 60DGC, which cools to a white, semi-solid, thermoreversible gel at room temperature, are described. The addition of an aqueous phase (water or niosome suspensions) up to 17% v/v to the oil phase at 60DGC produced water-in-oil and vesicle-in-water-in-oil systems, respectively. The release rate of hydrophilic solute from these gels was found to be lowest when a disperse system of spherical water droplets in the continuous oil phase was formed at high temperatures, compared to the faster release from the gel where the fibril structures acted as nearly continuous aqueous channels running through the organic medium, providing a means of traversing the oil phase.
Murdan S et al; S.T.P. Pharma Sci 6 (1): 44-8 (1996)
1. 1= PRACTICALLY NON-TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) ABOVE 15 G/KG, MORE THAN 1 QT FOR 70 KG PERSON (150 LB).
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-181
WHEN DIGESTED, BOTH THE FATTY ACID AND THE POLYHYDRIC ALCOHOL SORBITAN ARE ABSORBED, BUT THE LATTER IS COMPLETELY EXCRETED IN URINE.
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-181
