


1. 3-glcglc-20-glcglc-ginsenoside
2. Arasaponin E1
3. Ginsenoside-rb1
4. Gynosaponin C
5. Gypenoside Iii
6. Notoginsenoside Rb1
7. Panax Saponin E
8. Panaxoside Rb1
9. Pseudoginsenoside D
10. Sanchinoside E1
11. Sanchinoside Rb1
1. Gynosaponin C
2. 41753-43-9
3. Gypenoside Iii
4. Sanchinoside E1
5. Arasaponin E1
6. Panax Saponin E
7. Pseudoginsenoside D
8. Ginsenoside-rb1
9. Panaxsaponin E
10. (20s)-ginsenoside Rb1
11. Chembl501515
12. Grb 1
13. Chebi:67989
14. Sanchinoside Rb1
15. 7413s0wmh6
16. Notoginsenoside Rb1
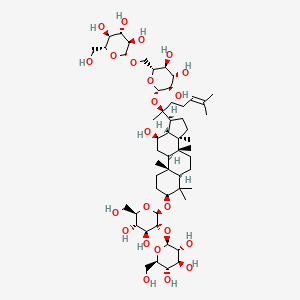
17. 2-o-beta-glucopyranosyl-(3beta,12beta)-20-[(6-o-beta-d-glucopyranosyl-beta-d-glucopyranosyl)oxy]-12-hydroxydammar-24-en-3-yl Beta-d-glucopyranoside
18. Ginsenosiderb1
19. Einecs 255-532-8
20. Mfcd00133367
21. Panaxoside Rb1
22. 2-o-beta-glucopyranosyl-(3beta,12beta)-20-((6-o-beta-d-glucopyranosyl-beta-d-glucopyranosyl)oxy)-12-hydroxydammar-24-en-3-yl-beta-d-glucopyranoside
23. Unii-7413s0wmh6
24. Gypenoside Cento
25. Nsc-310103
26. Nsc 310103
27. Gsrb1
28. Gs-rb1
29. 20(s)-ginsenoside Rb1
30. Ginsenoside Rb1 - 94%
31. Bidd:er0108
32. Ginsenoside Rb1 [usp-rs]
33. Ginsenoside Rb1 [who-dd]
34. Dtxsid401316929
35. Hms3885o12
36. Ex-a6786
37. Hy-n0039
38. Bdbm50317541
39. S3924
40. Akos025311537
41. Ccg-270640
42. Cs-3829
43. Db06749
44. Ncgc00347398-04
45. Bs-32417
46. Xg164977
47. N1620
48. C20713
49. 753g439
50. Q-100470
51. Ginsenoside Rb1, Primary Pharmaceutical Reference Standard
52. Ginsenoside Rb1, European Pharmacopoeia (ep) Reference Standard
53. Ginsenoside-rb1 From Panax Ginseng (korean Ginseng) Root, Triterpenoid Saponin
54. (3beta,12beta)-20-[(6-o-beta-d-glucopyranosyl-beta-d-glucopyranosyl)oxy]-12-hydroxydammar-24-en-3-yl 2-o-beta-d-glucopyranosyl-beta-d-glucopyranoside
55. (3beta,12beta)-20-{[6-o-(beta-d-glucopyranosyl)-beta-d-glucopyranosyl]oxy}-12-hydroxydammar-24-en-3-yl 2-o-beta-d-glucopyranosyl-beta-d-glucopyranoside
56. .beta.-d-glucopyranoside, (3.beta.,12.beta.)-20-((6-o-.beta.-d-glucopyranosyl-.beta.-d-glucopyranosyl)oxy)-12-hydroxydammar-24-en-3-yl 2-o-.beta.-d-glucopyranosyl-
57. 3beta-[beta-d-glucopyranosyl-(1->2)-beta-d Glucopyranosyloxy]-20-[beta-d-glucopyranosyl-(1->2)-beta-d Glucopyranosyloxy]dammar-24-en-12beta-ol
58. Beta-d-glucopyranoside, (3-beta,12-beta)-20-((6-o-beta-d-glucopyranosyl-beta-d-glucopyranosyl)oxy)-12-hydroxydammar-24-en-3-yl 2-o-beta-d-glucopyranosyl-
59. Ginsenoside Rb1 (constituent Of American Ginseng, Asian Ginseng, And Tienchi Ginseng) [dsc]
| Molecular Weight | 1109.3 g/mol |
|---|---|
| Molecular Formula | C54H92O23 |
| XLogP3 | 0.3 |
| Hydrogen Bond Donor Count | 15 |
| Hydrogen Bond Acceptor Count | 23 |
| Rotatable Bond Count | 16 |
| Exact Mass | 1108.60293918 g/mol |
| Monoisotopic Mass | 1108.60293918 g/mol |
| Topological Polar Surface Area | 377 Ų |
| Heavy Atom Count | 77 |
| Formal Charge | 0 |
| Complexity | 2000 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 30 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
