


1. Iressa
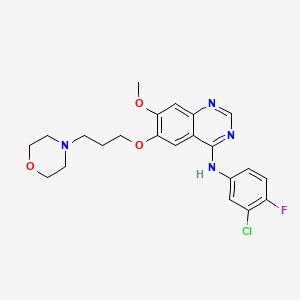
2. N-(3-chloro-4-fluorophenyl)-7-methoxy-6-(3-(4-morpholinyl)propoxy)-4-quinazolinamide
3. Zd 1839
4. Zd1839
1. 184475-35-2
2. Iressa
3. Zd1839
4. N-(3-chloro-4-fluorophenyl)-7-methoxy-6-(3-morpholinopropoxy)quinazolin-4-amine
5. Irressat
6. Zd 1839
7. Gefitinib (zd1839)
8. Zd-1839
9. N-(3-chloro-4-fluorophenyl)-7-methoxy-6-[3-(morpholin-4-yl)propoxy]quinazolin-4-amine
10. Gefitinib (gmp)
11. N-(3-chloro-4-fluoro-phenyl)-7-methoxy-6-(3-morpholin-4-ylpropoxy)quinazolin-4-amine
12. 4-(3'-chloro-4'-fluoroanilino)-7-methoxy-6-(3-morpholinopropoxy)quinazoline
13. N-(3-chloro-4-fluorophenyl)-7-methoxy-6-(3-morpholin-4-ylpropoxy)quinazolin-4-amine
14. Mfcd04307832
15. Chembl939
16. Nsc-759856
17. S65743jhbs
18. Chebi:49668
19. 3-chloro-4-fluoro-n-[(4z)-7-methoxy-6-(3-morpholin-4-ylpropoxy)quinazolin-4(1h)-ylidene]aniline
20. N-(3-chloro-4-fluorophenyl)-7-methoxy-6-[3-(4-morpholinyl)propoxy]-4-quinazolinamine
21. Gefitinib [usan]
22. Ncgc00159455-02
23. Dsstox_cid_21034
24. Dsstox_rid_79614
25. N-(3-chloro-4-fluoro-phenyl)-7-methoxy-6-(3-morpholinopropoxy)quinazolin-4-amine
26. Dsstox_gsid_41034
27. 4-quinazolinamine, N-(3-chloro-4-fluorophenyl)-7-methoxy-6-(3-(4-morpholinyl)propoxy)-
28. Iressa(tm)
29. Ire
30. Iressa (tn)
31. Ccris 9011
32. Cas-184475-35-2
33. Sr-00000000262
34. Gefitinibum
35. Unii-s65743jhbs
36. Gefitinib (jan/usan/inn)
37. Gefitinib [usan:inn:ban]
38. Gefitini; Iressa
39. 4-quinazolinamine, N-(3-chloro-4-fluorophenyl)-7-methoxy-6-[3-(4-morpholinyl)propoxy]-
40. N-(3-chloro-4-fluorophenyl)-7-methoxy-6-(3-(4-morpholinyl)propoxy)-4-quinazolinamine
41. Gefitinib (iressa)
42. Gefitinib - Iressa
43. Irressa
44. Iressa (astrazeneca)
45. Nchembio866-comp14
46. Kinome_3321
47. Kinome_3322
48. Gefitinib [inn]
49. Gefitinib [jan]
50. Gefitinib [mi]
51. Gefitinib [vandf]
52. Gefitinib [mart.]
53. Gefitinib [who-dd]
54. Schembl7866
55. Gefitinib,zd-1839,iressa
56. Gefitinib [ema Epar]
57. Kbioss_002241
58. Mls003899193
59. Cu-00000000396-1
60. Bdbm5447
61. Cid_123631
62. Gtpl4941
63. Gefitinib [orange Book]
64. Dtxsid8041034
65. Gefitinib [ep Monograph]
66. Gefitinib, >=98% (hplc)
67. Bcpp000221
68. Hms2089b19
69. Hms3244m21
70. Hms3244m22
71. Hms3244n21
72. Hms3295a21
73. Hms3413h08
74. Hms3654a07
75. Hms3677h08
76. Hms3714a05
77. Hms3748e17
78. Pharmakon1600-01502274
79. Bcp01365
80. Tox21_111683
81. Hy-50895g
82. Nsc715055
83. Nsc759856
84. Nsc800105
85. S1025
86. Stk621310
87. Zinc19632614
88. Akos000280752
89. Tox21_111683_1
90. Ab20814
91. Ac-1556
92. Bcp9000718
93. Ccg-220642
94. Cs-0124
95. Db00317
96. Ks-1204
97. Nsc 759856
98. Nsc-715055
99. Nsc-800105
100. 4-[(3-chloro-4-fluorophenyl)amino]-7-methoxy-6-(3-morpholinopropoxy)quinazoline
101. 4-quinazolinamine, N-(3-chloro-4-fluorophenyl)-7-methoxy-6-(3-4-morpholin)propoxy)-
102. 6-(3-morpholinopropoxy)-n-(3-chloro-4-fluorophenyl)-7-methoxyquinazolin-4-amine
103. Ncgc00159455-03
104. Ncgc00159455-04
105. Ncgc00159455-05
106. Ncgc00159455-06
107. Ncgc00159455-08
108. Ncgc00159455-09
109. Ncgc00159455-14
110. Bcb03_000781
111. Bg164498
112. Hy-50895
113. Smr002204119
114. Sy002154
115. Am20090619
116. Cs-0622782
117. Ft-0602325
118. G0546
119. Sw199108-4
120. Ec-000.2409
121. D01977
122. G-4408
123. K00240
124. Ab01273954-01
125. Ab01273954-02
126. Ab01273954-03
127. Ab01273954_04
128. 475g352
129. A812870
130. Q417824
131. Q-201149
132. Sr-00000000262-2
133. Sr-00000000262-3
134. Gefitinib, Europepharmacopoeia (ep) Reference Standard
135. Z1551429741
136. 4-(3'-chloro-4'-fluoroanilino)-7-methoxy-6-(3-morpholinopropoxy)-quinazoline
137. Gefitinib For System Suitability, Europepharmacopoeia (ep) Reference Standard
138. (3-chloro-4-fluoro-phenyl)-[7-methoxy-6-(3-morpholin-4-yl-propoxy)-quinazolin-4-yl]-amine
| Molecular Weight | 446.9 g/mol |
|---|---|
| Molecular Formula | C22H24ClFN4O3 |
| XLogP3 | 4.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 8 |
| Exact Mass | 446.1520965 g/mol |
| Monoisotopic Mass | 446.1520965 g/mol |
| Topological Polar Surface Area | 68.7 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 545 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the continued treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of either platinum-based or docetaxel chemotherapies.
FDA Label
Iressa is indicated for the treatment of adult patients with locally advanced or metastatic non-small-cell lung cancer with activating mutations of epidermal-growth-factor-receptor tyrosine kinase.
Gefitinib inhibits the intracellular phosphorylation of numerous tyrosine kinases associated with transmembrane cell surface receptors, including the tyrosine kinases associated with the epidermal growth factor receptor (EGFR-TK). EGFR is expressed on the cell surface of many normal cells and cancer cells.
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Protein Kinase Inhibitors
Agents that inhibit PROTEIN KINASES. (See all compounds classified as Protein Kinase Inhibitors.)
L01XE02
L01XE02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01E - Protein kinase inhibitors
L01EB - Epidermal growth factor receptor (egfr) tyrosine kinase inhibitors
L01EB01 - Gefitinib
Absorption
Absorbed slowly after oral administration with a mean bioavailability of 60%. Peak plasma levels occurs 3-7 hours post-administration. Food does not affect the bioavailability of gefitinib.
Route of Elimination
Elimination is by metabolism (primarily CYP3A4) and excretion in feces. Excretion is predominantly via the feces (86%), with renal elimination of drug and metabolites accounting for less than 4% of the administered dose.
Volume of Distribution
1400 L [IV administration]
Clearance
595 mL/min [IV administration]
Primarily hepatic via CYP3A4. Three sites of biotransformation have been identified: metabolism of the N-propoxymorpholino-group, demethylation of the methoxy-substituent on the quinazoline, and oxidative defluorination of the halogenated phenyl group.
Gefitinib has known human metabolites that include 4-Defluoro-4-hydroxy Gefitinib and O-Desmethyl Gefitinib.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
48 hours [IV administration]
Gefitinib is an inhibitor of the epidermal growth factor receptor (EGFR) tyrosine kinase that binds to the adenosine triphosphate (ATP)-binding site of the enzyme. EGFR is often shown to be overexpressed in certain human carcinoma cells, such as lung and breast cancer cells. Overexpression leads to enhanced activation of the anti-apoptotic Ras signal transduction cascades, subsequently resulting in increased survival of cancer cells and uncontrolled cell proliferation. Gefitinib is the first selective inhibitor of the EGFR tyrosine kinase which is also referred to as Her1 or ErbB-1. By inhibiting EGFR tyrosine kinase, the downstream signaling cascades are also inhibited, resulting in inhibited malignant cell proliferation.
