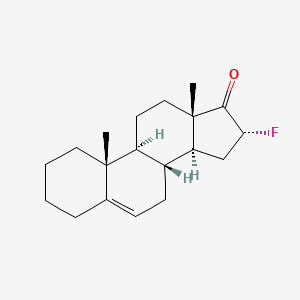

1. 5-androstene-16-fluoro-17-one
2. 5-androstene-16alpha-fluoro-17-one
3. Dhea Analog 8354
4. He2500
1. 112859-71-9
2. R7m5ugd04g
3. 156680-74-9
4. Chembl4441355
5. 16alpha-fluoro-5-androsten-17-one
6. (8r,9s,10r,13s,14s,16r)-16-fluoro-10,13-dimethyl-1,2,3,4,7,8,9,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-17-one
7. 16-fluoro-10,13-dimethyl-1,2,3,4,7,8,9,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-17-one
8. 16-fluoro-5-androsten-17-one
9. He2500
10. Unii-r7m5ugd04g
11. Ccris 4216
12. 8354 Compound
13. Schembl1418930
14. Niosh/bv8387230
15. Dtxsid90920920
16. Zinc3778181
17. Bdbm50507597
18. 16-alpha-fluoro-5-androsten-17-one
19. Db06250
20. Bv83872300
21. 16.alpha.-fluoroandrost-5-en-17-one
22. Androst-5-en-17-one, 16-fluoro-, (16alpha)-
23. Androst-5-en-17-one, 16-fluoro-, (16-alpha)-
24. J-002855
25. Androst-5-en-17-one, 16-fluoro-, (16.alpha.)-
26. Q27287895
27. (2r,3as,3br,9ar,9bs,11as)-2-fluoro-9a,11a-dimethyl-1h,2h,3h,3ah,3bh,4h,6h,7h,8h,9h,9ah,9bh,10h,11h,11ah-cyclopenta[a]phenanthren-1-one
| Molecular Weight | 290.4 g/mol |
|---|---|
| Molecular Formula | C19H27FO |
| XLogP3 | 5.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 290.20459364 g/mol |
| Monoisotopic Mass | 290.20459364 g/mol |
| Topological Polar Surface Area | 17.1 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 510 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in psoriasis and psoriatic disorders, hyperlipidemia, metabolic disease, cancer/tumors (unspecified), and obesity.
Fluasterone is a synthetically stable adrenocortical steroid fluorinated analogue of dehydroepiandrosterone (DHEA), a powerful anti-inflammatory molecule with androgenic or estrogenic side effects. It is proposed that fluasterone inhibits NF-kB activation and reduces oxidative stress, but other mechanisms may play a role. Fluasterone suppresses inflammation and is effective in preclinical models of chronic inflammatory disease including psoriasis, asthma, rheumatoid arthritis, multiple sclerosis and lupus erythematosus. Fluasterone has anti-inflammatory effects inpreclinical models of chronic inflammatory disease including psoriasis, asthma, rheumatoid arthritis, multiple sclerosis and lupus erythematosus. [Aeson Pharmaceuticals Executive Report]
