

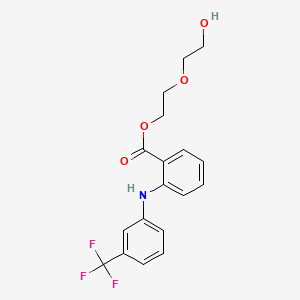

1. 2-(2-hydroxyethoxy)-ethyl-n-(alpha,alpha,alpha-trifluoro- M-tolyl)anthranilate
2. Algesalona E
3. Aspitopic
4. Bayro
5. Flogoprofen
6. Rheuma-gel-ratiopharm
7. Rheumon
8. Traumon
9. Zenavan
1. 30544-47-9
2. Bay D 1107
3. 2-(2-hydroxyethoxy)ethyl 2-((3-(trifluoromethyl)phenyl)amino)benzoate
4. 2-(2-hydroxyethoxy)ethyl 2-[3-(trifluoromethyl)anilino]benzoate
5. Tvx485
6. 2-(2-hydroxyethoxy)ethyl 2-{[3-(trifluoromethyl)phenyl]amino}benzoate
7. Whr-5020
8. Bayrogel
9. Rheumon
10. Kzf0xm66jc
11. Bayd-1107
12. Tvx-485
13. Bay-d-1107
14. 2-(2-hydroxyethoxy)ethyl-n-(alpha,alpha,alpha-trifluoro-m-tolyl)anthranilate
15. Benzoic Acid, 2-((3-(trifluoromethyl)phenyl)amino)-, 2-(2-hydroxyethoxy)ethyl Ester
16. Ncgc00016804-01
17. Rheumon Gel
18. Cas-30544-47-9
19. Benzoic Acid, 2-[[3-(trifluoromethyl)phenyl]amino]-, 2-(2-hydroxyethoxy)ethyl Ester
20. Dsstox_cid_25448
21. Dsstox_rid_80887
22. Dsstox_gsid_45448
23. Etofenamato
24. Etofenamatum
25. Etofenamatum [inn-latin]
26. Etofenamato [inn-spanish]
27. Tvx 485
28. Whr 5020
29. Einecs 250-231-8
30. Unii-kzf0xm66jc
31. Brn 2953263
32. Etofenamate [usan:inn:ban]
33. 2-(2-hydroxyethoxy)ethyl Fufenamate
34. 2-(2-hydroxyaethoxy)aethylester Der Flutenaminsaeure [german]
35. Etofenamate [mi]
36. Prestwick0_001014
37. Prestwick1_001014
38. Prestwick2_001014
39. Etofenamate [inn]
40. Etofenamate [jan]
41. Etofenamate (usan/inn)
42. Etofenamate [usan]
43. 2-(2-hydroxyethoxy)ethyl N-(alpha,alpha,alpha-trifluoro-m-tolyl)anthranilate
44. Etofenamate [mart.]
45. Schembl25152
46. Etofenamate [who-dd]
47. 2-(2-hydroxyaethoxy)aethylester Der Flutenaminsaeure
48. Spbio_003038
49. Chembl1451633
50. Dtxsid2045448
51. Chebi:94731
52. Ex-a275
53. Etofenamate [ep Monograph]
54. Etofenamate For Peak Identification
55. Hms1571k09
56. Hms3713h06
57. Bcp08746
58. Zinc2034516
59. Tox21_110618
60. Mfcd00242806
61. S6742
62. Akos026750534
63. Tox21_110618_1
64. Ccg-220592
65. Cs-0905
66. Db08984
67. Anthranilic Acid, N-(alpha,alpha,alpha-trifluoro-m-tolyl)-, 2-(2-hydroxyethoxy)ethyl Ester
68. Ncgc00016804-02
69. Ncgc00016804-03
70. Ac-35208
71. As-73948
72. Da-33632
73. Hy-17361
74. Ft-0668428
75. D04102
76. 544e479
77. Q414378
78. Sr-01000872640
79. J-018005
80. Sr-01000872640-1
81. Brd-k73541271-001-01-0
82. Z1248763315
83. 2-[3-(trifluoromethyl)anilino]benzoic Acid 2-(2-hydroxyethoxy)ethyl Ester
84. Tv-485;tv485;tv 485; Whr-5020; Whr 5020; Whr5020
85. 2-(2-hydroxyethoxy)ethyl N-(.alpha.,.alpha.,.alpha.-trifluoro-m-tolyl)anthranilate
| Molecular Weight | 369.3 g/mol |
|---|---|
| Molecular Formula | C18H18F3NO4 |
| XLogP3 | 4.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 9 |
| Exact Mass | 369.11879254 g/mol |
| Monoisotopic Mass | 369.11879254 g/mol |
| Topological Polar Surface Area | 67.8 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 433 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
M02AA06
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
M - Musculo-skeletal system
M02 - Topical products for joint and muscular pain
M02A - Topical products for joint and muscular pain
M02AA - Antiinflammatory preparations, non-steroids for topical use
M02AA06 - Etofenamate
