

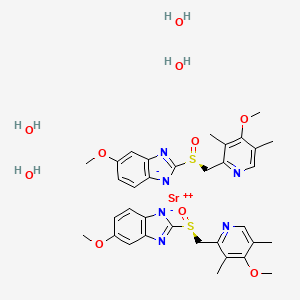

1. Esomeprazole
2. Esomeprazole Magnesium
3. Esomeprazole Potassium
4. Esomeprazole Sodium
5. Esomeprazole Strontium Anhydrous
6. Nexium
7. Strontium, Esomeprazole
1. S-omeprazole Strontium Hydrate
2. Fm0f67
3. Esomeprazole Strontium [usan]
4. 934714-36-0
5. Fm-0f67
6. C5n25h3803
7. (-)-5-methoxy-2-((s)-((4-methoxy-3,5-dimethylpyridin-2-yl)methyl)sulfinyl)-1h- Benzimidazole Strontium Salt Tetrahydrate
8. 1h-benzimidazole, 6-methoxy-2-((s)-((4-methoxy-3,5-dimethyl-2-pyridinyl) Methyl)sulfinyl)-, Strontium Salt, Hydrate (2:1:4)
9. Esomeprazole Strontium (usan)
10. Strontium;5-methoxy-2-[(s)-(4-methoxy-3,5-dimethylpyridin-2-yl)methylsulfinyl]benzimidazol-1-ide;tetrahydrate
11. Unii-c5n25h3803
12. Esomeprazole Strontium (tn)
13. Esomeprazole Strontium Hydrate
14. Chembl2146140
15. Hip1601
16. Esomeprazole Strontium Tetrahydrate
17. Hip-1601
18. Esomeprazole Strontium [vandf]
19. Esomeprazole Strontium [usp-rs]
20. Esomeprazole Strontium [who-dd]
21. Esomeprazole Strontium [orange Book]
22. Esomeprazole Strontium [usp Monograph]
23. D10120
24. Q27275225
| Molecular Weight | 848.5 g/mol |
|---|---|
| Molecular Formula | C34H44N6O10S2Sr |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 18 |
| Rotatable Bond Count | 10 |
| Exact Mass | 848.16164622 g/mol |
| Monoisotopic Mass | 848.16164622 g/mol |
| Topological Polar Surface Area | 167 Ų |
| Heavy Atom Count | 53 |
| Formal Charge | 0 |
| Complexity | 453 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 7 |
| 1 of 2 | |
|---|---|
| Drug Name | Esomeprazole strontium |
| PubMed Health | Esomeprazole |
| Drug Classes | Gastric Acid Secretion Inhibitor, Gastrointestinal Agent |
| Drug Label | The active ingredient in the proton pump inhibitor esomeprazole strontium delayed-release capsules is bis(5methoxy-2-[(S)-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole-1-yl)strontium tetrahydrate. Esomeprazole is the S... |
| Active Ingredient | Esomeprazole strontium |
| Dosage Form | Capsule, delayed release |
| Route | Oral |
| Strength | eq 40mg base; eq 20mg base |
| Market Status | Prescription |
| Company | Hanmi Pharm |
| 2 of 2 | |
|---|---|
| Drug Name | Esomeprazole strontium |
| PubMed Health | Esomeprazole |
| Drug Classes | Gastric Acid Secretion Inhibitor, Gastrointestinal Agent |
| Drug Label | The active ingredient in the proton pump inhibitor esomeprazole strontium delayed-release capsules is bis(5methoxy-2-[(S)-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole-1-yl)strontium tetrahydrate. Esomeprazole is the S... |
| Active Ingredient | Esomeprazole strontium |
| Dosage Form | Capsule, delayed release |
| Route | Oral |
| Strength | eq 40mg base; eq 20mg base |
| Market Status | Prescription |
| Company | Hanmi Pharm |
Anti-Ulcer Agents
Various agents with different action mechanisms used to treat or ameliorate PEPTIC ULCER or irritation of the gastrointestinal tract. This has included ANTIBIOTICS to treat HELICOBACTER INFECTIONS; HISTAMINE H2 ANTAGONISTS to reduce GASTRIC ACID secretion; and ANTACIDS for symptomatic relief. (See all compounds classified as Anti-Ulcer Agents.)
Proton Pump Inhibitors
Compounds that inhibit H(+)-K(+)-EXCHANGING ATPASE. They are used as ANTI-ULCER AGENTS and sometimes in place of HISTAMINE H2 ANTAGONISTS for GASTROESOPHAGEAL REFLUX. (See all compounds classified as Proton Pump Inhibitors.)
