


1. Viberzi
1. 864821-90-9
2. Viberzi
3. Truberzi
4. Jnj-27018966
5. Eluxadoline [usan]
6. Jnj 27018966
7. 45tpj4mbq1
8. Chembl2159122
9. Chebi:85980
10. 864821-90-9 (free Base)
11. Eluxadoline (usan)
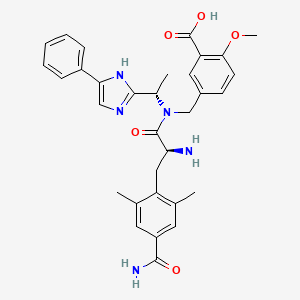
12. 5-(((s)-2-amino-3-(4-carbamoyl-2,6-dimethylphenyl)-n-((s)-1-(5-phenyl-1h-imidazol-2-yl)ethyl)propanamido)methyl)-2-methoxybenzoic Acid
13. 5-({(4-carbamoyl-2,6-dimethyl-l-phenylalanyl)[(1s)-1-(4-phenyl-1h-imidazol-2-yl)ethyl]amino}methyl)-2-methoxybenzoic Acid
14. Benzoic Acid, 5-((((2s)-2-amino-3-(4-(aminocarbonyl)-2,6-dimethylphenyl)-1-oxopropyl)((1s)-1-(5-phenyl-1h-imidazol-2-yl)ethyl)amino)methyl)-2-methoxy-
15. Eluxadoline [usan:inn]
16. Unii-45tpj4mbq1
17. Benzoic Acid, 5-[[[(2s)-2-amino-3-[4-(aminocarbonyl)-2,6-dimethylphenyl]-1-oxopropyl][(1s)-1-(5-phenyl-1h-imidazol-2-yl)ethyl]amino]methyl]-2-methoxy-
18. Viberzi (tn)
19. Eluxadoline [mi]
20. Eluxadoline [inn]
21. Eluxadoline [who-dd]
22. Gtpl7691
23. Dea No. 9725
24. Eluxadoline [nflis-drug]
25. Schembl12971682
26. Schembl17950908
27. Dtxsid70235589
28. Eluxadoline [orange Book]
29. Amy39829
30. Ex-a1169
31. Mfcd28386164
32. Zinc14210876
33. Akos030632800
34. Ccg-270093
35. Cs-3855
36. Db09272
37. Jnj27018966
38. Ncgc00485958-01
39. Ac-30329
40. As-35135
41. Hy-12247
42. Ft-0701295
43. J3.535.064k
44. A14085
45. D10403
46. Q20539232
47. 5-((((2s)-2-amino-3-(4-carbamoyl-2,6-dimethylphenyl)propanoyl)((1s)-1-(4-phenyl-1h-imidazol-2-yl)ethyl)amino(methyl)-2-methoxybenzoic Acid
48. 5-(((s)-2-amino-3-(4-carbamoyl-2,6-dimethylphenyl)-n-((s)-1-(4-phenyl-1h-imidazol-2-yl)ethyl)propanamido)methyl)-2-methoxybenzoic Acid
49. 5-((2-amino-3-(4-carbamoyl-2,6-dimethylphenyl)-n-(1-(5-phenyl-1h-imidazol-2-yl)ethyl)propanamido)methyl)-2-methoxybenzoic Acid
50. 5-[[[(2s)-2-amino-3-(4-carbamoyl-2,6-dimethylphenyl)propanoyl]-[(1s)-1-(4-phenyl-3h-imidazol-2-yl)ethyl]amino]methyl]-2-methoxybenzoic Acid
51. 5-[[[(2s)-2-amino-3-(4-carbamoyl-2,6-dimethylphenyl)propanoyl]-[(1s)-1-(5-phenyl-1h-imidazol-2-yl)ethyl]amino]methyl]-2-methoxybenzoic Acid
| Molecular Weight | 569.6 g/mol |
|---|---|
| Molecular Formula | C32H35N5O5 |
| XLogP3 | 0.9 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 11 |
| Exact Mass | 569.26381923 g/mol |
| Monoisotopic Mass | 569.26381923 g/mol |
| Topological Polar Surface Area | 165 Ų |
| Heavy Atom Count | 42 |
| Formal Charge | 0 |
| Complexity | 917 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of irritable bowel syndrome with diarrhea (IBS-D).
FDA Label
Truberzi is indicated in adults for the treatment of irritable bowel syndrome with diarrhoea (IBS D).
Gastrointestinal Agents
Drugs used for their effects on the gastrointestinal system, as to control gastric acidity, regulate gastrointestinal motility and water flow, and improve digestion. (See all compounds classified as Gastrointestinal Agents.)
A07
A - Alimentary tract and metabolism
A07 - Antidiarrheals, intestinal antiinflammatory/antiinfective agents
A07D - Antipropulsives
A07DA - Antipropulsives
A07DA06 - Eluxadoline
Absorption
The oral absorption of eluxadoline is poor - estimated to be 1.02%, this could be attributed to poor in vitro GI permeability, and its zwitterionic nature leading to a negatively charged molecule across the GI pH range.
Route of Elimination
82% excreted in feces, <1% excreted in urine.
The metabolism of eluxadoline is currently unclear, however evidence suggests limited glucoronidation forms an acyl glucuronide metabolite that is then excreted into urine.
The mean plasma elimination half-life ranged from 3.7 hours to 6 hours.
Eluxadoline is a mu-opioid receptor agonis, kappa opioid receptor agonist and a delta opioid receptor antagonist. Eluxadoline is used for diarrhea predominant IBS because it reduces intestinal contractility and normalizes stress-induced acceleration of upper GI transit. Antagonistic activity at the delta receptor minimizes the constipating effect usually seen by mu-opioid receptor agonists alone. Because of it's limited systemic bioavailability, there may be less side effects associated with the use of eluxadoline in comparison with other therapies used to treat diarrhea predominant IBS.
