

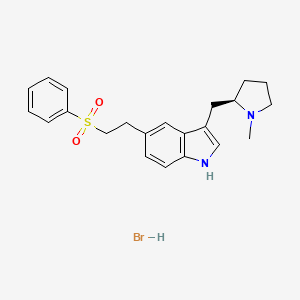

1. (r)-3-((1-methyl-2-pyrrolidinyl)methyl)-5-(2-(phenylsulfonyl)ethyl)-1h-indole
2. (r)-3-((1-methyl-2-pyrrolidinyl)methyl)-5-(2-(phenylsulfonyl)ethyl)-1h-indole Monohydrobromide
3. 3-(1-methyl-2-pyrrolidinylmethyl)-5-(2-(phenylsulfonyl)ethyl)-1h-indole Hydrobromide
4. Eletriptan
5. Relpax
6. Uk 166,044
7. Uk 166044
8. Uk-116,044-04
9. Uk-116044-04
10. Uk-166,044
11. Uk-166044
1. 177834-92-3
2. Eletriptan Hbr
3. Relpax
4. Eletriptan Hydrobromide [usan]
5. Eletriptan (hydrobromide)
6. (r)-3-((1-methylpyrrolidin-2-yl)methyl)-5-(2-(phenylsulfonyl)ethyl)-1h-indole Hydrobromide
7. Eletriptan Monohydrobromide
8. Uk 116044-04
9. Chebi:61176
10. M41w832ta3
11. Uk-116,044-04
12. (r)-3-((1-methyl-2-pyrrolidinyl)methyl)-5-(2-(phenylsulfonyl)ethyl)-1h-indole Monohydrobromide
13. 5-[2-(benzenesulfonyl)ethyl]-3-[[(2r)-1-methylpyrrolidin-2-yl]methyl]-1h-indole;hydrobromide
14. Nsc-759258
15. 3-(((r)-1-methyl-2-pyrrolidinyl)methyl)-5-(2-(phenylsulfonyl)ethyl)indole, Monohydrobromide
16. 5-[2-(phenylsulfonyl)ethyl]-3-[(2r)-pyrrolidin-2-ylmethyl]-1h-indole Hydrobromide
17. C22h26n2o2s.hbr
18. Unii-m41w832ta3
19. Relpax (tn)
20. Schembl317370
21. Chembl1201003
22. Dtxsid001016113
23. Hy-a0010
24. Eletriptan Hydrobromide (jan/usan)
25. Eletriptan Hydrobromide [mi]
26. Tox21_500408
27. Eletriptan Hydrobromide [jan]
28. Mfcd08141806
29. S3180
30. Akos024262728
31. Ac-3398
32. Bcp9000640
33. Ccg-221712
34. Cs-0379
35. Eletriptan Hydrobromide [mart.]
36. Nsc 759258
37. 3-[[(2r)-1-methyl-2-pyrrolidinyl]methyl]-5-[2-(phenylsulfonyl)ethyl]-1h-indole Hydrobromide
38. Eletriptan Hydrobromide [usp-rs]
39. Eletriptan Hydrobromide [who-dd]
40. Ncgc00261093-01
41. Bs-42146
42. Eletriptan Hydrobromide, >=98% (hplc)
43. Eletriptan Hydrobromide [orange Book]
44. Sw220149-1
45. D01973
46. J-011323
47. J-520433
48. Q27130865
49. 3-(n-methyl-2(r)-pyrrolidinyl Methyl)-5-[2-(phenyl Sulfonyl)ethyl]-1h-indole Hydrobromide
50. 3-(n-methyl-2(r)-pyrrolidinylmethyl)-5-(2-phenylsulphonylethly)-1h-indole Hydrobromide
51. 3-(n-methyl-2(r)-pyrrolidinylmethyl)-5-(2-phenylsulphonylethyl)-1h-indole Hydrobromide
52. 3-(n-methyl-2(r)-pyrrolidinylmethyl)-5-[2-(phenyl Sulfonyl)ethyl]-1h-indole Hydrobromide
53. 3-{[1-methylpyrrolidin-2(r)-yl]methyl}-5-(2-phenylsulphonylethyl)-1h-indole Hydrobromide
54. (r)-3-[(1-methyl-2-pyrrolidinyl) Methyl]-5-[2-(phenylsulfonyl) Ethyl]-1h-indole Monohydrobromide
55. 1h-indole, 3-(((2r)-1-methyl-2-pyrrolidinyl))methyl)-5-(2-(phenylsulfonyl)ethyl)-, Monohydrobromide
56. 1h-indole, 3-[[(2r)-1-methyl-2-pyrrolidinyl]methyl]-5-[2-(phenylsulfonyl)ethyl]-, Hydrobromide (1:1)
57. 3-[[(2r)-1-methyl-2-pyrrolidinyl]me Thyl]-5-[2-(phenylsulfonyl)ethyl]-1h-indole Hydrobromide
58. 5-[2-(benzenesulfonyl)ethyl]-3-[[(2r)-1-methylpyrrolidin-2-yl]methyl]-1h-indol-1-ium;bromide
| Molecular Weight | 463.4 g/mol |
|---|---|
| Molecular Formula | C22H27BrN2O2S |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 6 |
| Exact Mass | 462.09766 g/mol |
| Monoisotopic Mass | 462.09766 g/mol |
| Topological Polar Surface Area | 61.6 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 582 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Eletriptan hydrobromide |
| Drug Label | RELPAX (eletriptan) Tablets contain eletriptan hydrobromide, which is a selective 5-hydroxytryptamine 1B/1D (5-HT1B/1D) receptor agonist. Eletriptan is chemically designated as (R)-3-[(1-Methyl-2-pyrrolidinyl)methyl]-5-[2-(phenylsulfonyl)ethyl]-1H-... |
| Active Ingredient | Eletriptan hydrobromide |
| Dosage Form | Tablet |
| Route | oral |
| Strength | eq 40mg base; eq 20mg base; 40mg; 20mg |
| Market Status | Tentative Approval |
| Company | Apotex; Teva Pharms Usa |
| 2 of 2 | |
|---|---|
| Drug Name | Eletriptan hydrobromide |
| Drug Label | RELPAX (eletriptan) Tablets contain eletriptan hydrobromide, which is a selective 5-hydroxytryptamine 1B/1D (5-HT1B/1D) receptor agonist. Eletriptan is chemically designated as (R)-3-[(1-Methyl-2-pyrrolidinyl)methyl]-5-[2-(phenylsulfonyl)ethyl]-1H-... |
| Active Ingredient | Eletriptan hydrobromide |
| Dosage Form | Tablet |
| Route | oral |
| Strength | eq 40mg base; eq 20mg base; 40mg; 20mg |
| Market Status | Tentative Approval |
| Company | Apotex; Teva Pharms Usa |
Serotonin Receptor Agonists
Endogenous compounds and drugs that bind to and activate SEROTONIN RECEPTORS. Many serotonin receptor agonists are used as ANTIDEPRESSANTS; ANXIOLYTICS; and in the treatment of MIGRAINE DISORDERS. (See all compounds classified as Serotonin Receptor Agonists.)
