

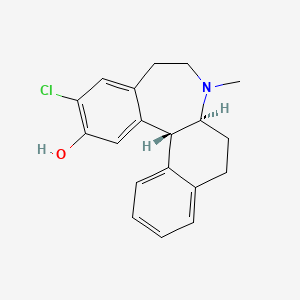

1. 6,7,7a,8,9,13b-hexahydro-3-chloro-2-hydroxy-n-methyl-5h-benzo(d)naphtho-(2,1b)azepine
2. Sch 39166
3. Sch-39166
4. Sch39166
1. 112108-01-7
2. Sch-39166
3. Ecopipam [inn]
4. Sch 39166
5. Sch39166
6. Chembl298406
7. 0x748o646k
8. 5h-benzo(d)naphth(2,1-b)azepin-12-ol, 11-chloro-6,6a,7,8,9,13b-hexahydro-7-methyl-, (6as,13br)-
9. Dsstox_cid_23814
10. Dsstox_rid_80075
11. Dsstox_gsid_43814
12. (-)-(6as,13br)-11-chloro-6,6a,7,8,9,13b-hexahydro-7-methyl-5h-benzo(d)naphth(2,1-b)azepin-12-ol
13. (6as,13br)-11-chloro-7-methyl-6,6a,7,8,9,13b-hexahydro-5h-benzo[d]naphtho[2,1-b]azepin-12-ol
14. Cas-112108-01-7
15. Ncgc00092362-01
16. Unii-0x748o646k
17. Ecopipam [who-dd]
18. 6,7,7a,8,9,13b-hexahydro-3-chloro-2-hydroxy-n-methyl-5h-benzo(d)naphtho(2,1b)azepine
19. 5h-benzo(d)naphth(2,1-b)azepin-12-ol, 11-chloro-6,6a,7,8,9,13b-hexahydro-7-methyl-, Trans-(-)-
20. Psyrx-101
21. Gtpl3304
22. Schembl1649794
23. Zinc3897
24. Dtxsid8043814
25. Chebi:93645
26. 11-chloro-7-methyl-5,6a,7,8,9,13b-hexahydro-6h-7-aza-benzo[6,7]cyclohepta[1,2-a]naphthalen-12-ol
27. Tox21_111197
28. Bdbm50004823
29. Tox21_111197_1
30. Db12273
31. (6as,13br)-11-chloro-7-methyl-5,6,6a,8,9,13b-hexahydronaphtho[1,2-a][3]benzazepin-12-ol
32. Ncgc00092362-02
33. Hy-14690
34. Cs-0003511
35. Q5333851
36. Brd-k94270326-004-01-0
37. (6as,13br)-11-chloro-7-methyl-5,6a,7,8,9,13b-hexahydro-6h-7-aza-benzo[6,7]cyclohepta[1,2-a]naphthalen-12-ol
38. Trans-(-) 11-chloro-7-methyl-5,6a,7,8,9,13b-hexahydro-6h-7-aza-benzo[6,7]cyclohepta[1,2-a]naphthalen-12-ol
| Molecular Weight | 313.8 g/mol |
|---|---|
| Molecular Formula | C19H20ClNO |
| XLogP3 | 4.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 313.1233420 g/mol |
| Monoisotopic Mass | 313.1233420 g/mol |
| Topological Polar Surface Area | 23.5 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 403 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antipsychotic Agents
Agents that control agitated psychotic behavior, alleviate acute psychotic states, reduce psychotic symptoms, and exert a quieting effect. They are used in SCHIZOPHRENIA; senile dementia; transient psychosis following surgery; or MYOCARDIAL INFARCTION; etc. These drugs are often referred to as neuroleptics alluding to the tendency to produce neurological side effects, but not all antipsychotics are likely to produce such effects. Many of these drugs may also be effective against nausea, emesis, and pruritus. (See all compounds classified as Antipsychotic Agents.)
Dopamine Antagonists
Drugs that bind to but do not activate DOPAMINE RECEPTORS, thereby blocking the actions of dopamine or exogenous agonists. Many drugs used in the treatment of psychotic disorders (ANTIPSYCHOTIC AGENTS) are dopamine antagonists, although their therapeutic effects may be due to long-term adjustments of the brain rather than to the acute effects of blocking dopamine receptors. Dopamine antagonists have been used for several other clinical purposes including as ANTIEMETICS, in the treatment of Tourette syndrome, and for hiccup. Dopamine receptor blockade is associated with NEUROLEPTIC MALIGNANT SYNDROME. (See all compounds classified as Dopamine Antagonists.)
