


1. Anectine
2. Bromide, Suxamethonium
3. Celocurine
4. Dibromide, Succinylcholine
5. Dichloride, Succinylcholine
6. Dicholine Succinate
7. Diiodide, Succinylcholine
8. Diperchlorate, Succinylcholine
9. Ditilin
10. Listenon
11. Lysthenon
12. Myorelaxin
13. Quelicin
14. Succicuran
15. Succinate, Dicholine
16. Succinylcholine
17. Succinylcholine Dibromide
18. Succinylcholine Dichloride
19. Succinylcholine Dichloride, Di H2o
20. Succinylcholine Dichloride, Di-h2o
21. Succinylcholine Diiodide
22. Succinylcholine Diperchlorate
23. Succinylcholine Iodide
24. Succinyldicholine
25. Suxamethonium
26. Suxamethonium Bromide
27. Suxamethonium Chloride
1. 71-27-2
2. Suxamethonium Chloride
3. Succinyldicholine Chloride
4. Anectine
5. Suxamethonii Chloridum
6. Lysthenon
7. Midarine
8. Quelicin
9. Scoline
10. Pantolax
11. Suxamethonium Dichloride
12. Chlorsuccinylcholin
13. Succinylcholine Dichloride
14. Succinylforte
15. Lysthenone
16. Succicuran
17. Sucostrin
18. Suxcert
19. Suxinyl
20. Bis(succinyldichlorocholine)
21. Choline Chloride Succinate (2:1)
22. Succinyl-asta
23. Scoline Chloride
24. Cloruro De Suxametonio
25. Anectine Chloride
26. Quelicin Chloride
27. Sucostrin Chloride
28. Chlorure De Succinilcoline
29. 2-dimethylaminoethyl Succinate Dimethochloride
30. Suxamethionium Chloride
31. Listenon
32. Succinoylcholine Chloride
33. Choline, Chloride Succinate
34. Choline Succinate Dichloride
35. Succinyl Bischoline Chloride
36. Succinylbischoline Dichloride
37. (2-hydroxyethyl)trimethylammonium Chloride Succinate
38. I9l0ddd30i
39. Bis(2-dimethylaminoethyl)succinate Bis(methochloride)
40. Suxamethonium Chloride [inn]
41. Chebi:61219
42. Nsc-49132
43. Succinic Acid, Diester With Choline Chloride
44. Ncgc00094357-01
45. Myoplegine
46. Sukolin
47. Succin
48. Dsstox_cid_3603
49. Diacetylcholine Dichloride
50. Dsstox_rid_77103
51. Ethanaminium, 2,2'-((1,4-dioxo-1,4-butanediyl)bis(oxy))bis(n,n,n-trimethyl-), Dichloride
52. Trimethyl-[2-[4-oxo-4-[2-(trimethylazaniumyl)ethoxy]butanoyl]oxyethyl]azanium;dichloride
53. Dsstox_gsid_23603
54. Suxamethonium Chloride (inn)
55. Succinyldicholine Dichloride
56. Succamethonium Chloratum
57. Quelicin Preservative Free
58. 2,2'-[(1,4-dioxobutane-1,4-diyl)bis(oxy)]bis(n,n,n-trimethylethanaminium) Dichloride
59. Cas-71-27-2
60. Chlorsuccinylcholin [german]
61. Sukcinylcholinchlorid [czech]
62. Sukcinylcholinchlorid
63. Succinilcolina Cloruro [dcit]
64. Choline, Succinyl-, Dichloride
65. Succinilcolina Cloruro
66. Succinylcholine Chloride [usan]
67. Chlorure De Succinilcoline [french]
68. Cloruro Di Succinilcolina
69. Cloruro Di Succinilcolina [italian]
70. Suxamethonii Chloridum [inn-latin]
71. Einecs 200-747-4
72. Nsc 49132
73. Cloruro De Suxametonio [inn-spanish]
74. Unii-i9l0ddd30i
75. Ai3-51679
76. Anectine (tn)
77. Quelicin (tn)
78. Choline, Chloride, Succinate (2:1)
79. Mfcd00038725
80. Succinylcholine Chloride [usan:usp]
81. Suxamethone
82. Choline, Hydroxide, Succinate (2:1), Dihydrochloride
83. Succinyl-bis(n-(2-oxyethyl)trimethylammonium)-dichlorid
84. 3,8-dioxa-4,7-dioxodercan-1,10-bis(trimethylammonium)dichlorid
85. Succinic Acid, Bis(beta-dimethylaminoethyl) Ester, Dimethochloride
86. Chembl983
87. Schembl41536
88. Anhydrous Suxamethonium Chloride
89. Succinylcholine Chloride (usp)
90. Dtxsid1023603
91. Anhydrous Succinylcholine Chloride
92. Suxamethonium Chloride (anhydrous)
93. Hms3263g22
94. Anhydrous Succinylcholine Dichloride
95. Succinylcholine Chloride (anhydrous)
96. Tox21_111268
97. Tox21_501080
98. Anhydrous Succinyldicholine Dichloride
99. Succinylcholine Chloride [mi]
100. Succinylcholine Dichloride (anhydrous)
101. Akos015961173
102. Suxamethonium Chloride [mart.]
103. Tox21_111268_1
104. Ac-4688
105. Ccg-222384
106. Lp01080
107. Suxamethonium Chloride [who-dd]
108. Ethanaminium, 2,2'-((1,4-dioxo-1,4-butanediyl)bis(oxy))bis(n,n,n-trimethyl-, Dichloride
109. Succinylcholine Chloride [vandf]
110. Succinyldicholine Dichloride (anhydrous)
111. Ncgc00015971-05
112. Ncgc00261765-01
113. Succinylcholine Chloride [usp-rs]
114. Db-055512
115. Eu-0101080
116. Suxamethonium Chloride [ep Monograph]
117. Succinylcholine Chloride [orange Book]
118. D00766
119. S 8251
120. Succinylcholine Chloride [usp Monograph]
121. Sr-01000075346
122. Q-201750
123. Sr-01000075346-2
124. Q12455154
| Molecular Weight | 361.3 g/mol |
|---|---|
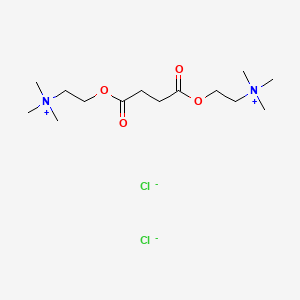
| Molecular Formula | C14H30Cl2N2O4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 11 |
| Exact Mass | 360.1582628 g/mol |
| Monoisotopic Mass | 360.1582628 g/mol |
| Topological Polar Surface Area | 52.6 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 284 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
| 1 of 2 | |
|---|---|
| Drug Name | Quelicin preservative free |
| Active Ingredient | Succinylcholine chloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 20mg/ml; 100mg/ml |
| Market Status | Prescription |
| Company | Hospira |
| 2 of 2 | |
|---|---|
| Drug Name | Quelicin preservative free |
| Active Ingredient | Succinylcholine chloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 20mg/ml; 100mg/ml |
| Market Status | Prescription |
| Company | Hospira |
Neuromuscular Depolarizing Agents
Drugs that interrupt transmission at the skeletal neuromuscular junction by causing sustained depolarization of the motor end plate. These agents are primarily used as adjuvants in surgical anesthesia to cause skeletal muscle relaxation. (See all compounds classified as Neuromuscular Depolarizing Agents.)
