


1. Dihydrochloride, Pirenzepine
2. Gastrotsepin
3. Gastrozepin
4. L-s 519
5. Ls 519
6. Ls-519
7. Ls519
8. Piren Basan
9. Piren-basan
10. Pirenzepin
11. Pirenzepin Ratiopharm
12. Pirenzepin Von Ct
13. Pirenzepin-ratiopharm
14. Pirenzepine Dihydrochloride
15. Pyrenzepine
16. Ulcoprotect
17. Ulgescum
18. Von Ct, Pirenzepin
1. 28797-61-7
2. Pirenzepinum
3. Pirenzepina
4. Pirenzepin
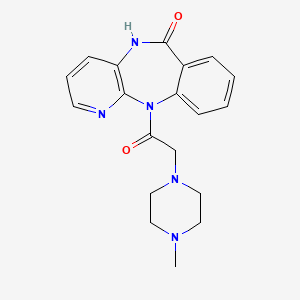
5. 11-[2-(4-methylpiperazin-1-yl)acetyl]-5h-pyrido[2,3-b][1,4]benzodiazepin-6-one
6. Pirenzepine (inn)
7. 11-((4-methyl-1-piperazinyl)acetyl)-5,11-dihydro-6h-pyrido(2,3-b)(1,4)benzodiazepin-6-one
8. 11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6h-pyrido[2,3-b][1,4]benzodiazepin-6-one
9. Chembl9967
10. 5,11-dihydro-11-((4-methyl-1-piperazinyl)acetyl)-6h-pyrido(2,3-b)(1,4)benzodiazepin-6-one
11. 6h-pyrido(2,3-b)(1,4)benzodiazepin-6-one, 5,11-dihydro-11-((4-methyl-1-piperazinyl)acetyl)-
12. Aci-91
13. Chebi:8247
14. Pirenzepinedihydrochloridemonohydrate
15. 3g0285n20n
16. Ncgc00015836-08
17. Pirenzepinum [inn-latin]
18. Pirenzepina [inn-spanish]
19. Pirenzepine [inn]
20. Dsstox_cid_3487
21. Pirenzepine [inn:ban]
22. Dsstox_rid_77049
23. Dsstox_gsid_23487
24. Mls000069702
25. 5,11-dihydro-11-[(4-methyl-1-piperazinyl)acetyl]-6h-pyrido[2,3-b][1,4]benzodiazepin-6-one
26. 6h-pyrido[2,3-b][1,4]benzodiazepin-6-one, 5,11-dihydro-11-[(4-methyl-1-piperazinyl)acetyl]-
27. 6h-pyrido[2,3-b][1,4]benzodiazepin-6-one, 5,11-dihydro-11-[(4-methyl-1-piperazinyl)acetyl]- (8ci,9ci)
28. 6h-pyrido[2,3-b][1,4]benzodiazepin-6-one, 5,11-dihydro-11-[2-(4-methyl-1-piperazinyl)acetyl]-
29. Cas-28797-61-7
30. Smr000058502
31. Einecs 249-228-4
32. Ls-519
33. Brn 0628987
34. Unii-3g0285n20n
35. Pirenzepine-[d8]
36. Spectrum_001378
37. Tocris-1071
38. Starbld0016668
39. Pirenzepine [mi]
40. Prestwick0_000129
41. Prestwick1_000129
42. Prestwick2_000129
43. Prestwick3_000129
44. Spectrum2_001417
45. Spectrum3_001453
46. Spectrum4_000437
47. Spectrum5_001344
48. Lopac-p-7412
49. Pirenzepine [vandf]
50. Lopac0_000962
51. Schembl41705
52. Bspbio_000178
53. Bspbio_002945
54. Gtpl328
55. Kbiogr_000794
56. Kbioss_001858
57. Pirenzepine [who-dd]
58. Divk1c_000127
59. Spbio_001494
60. Spbio_002117
61. Bpbio1_000196
62. Cid_185248
63. Dtxsid7023487
64. Bdbm39341
65. Kbio1_000127
66. Kbio2_001858
67. Kbio2_004426
68. Kbio2_006994
69. Kbio3_002445
70. Ninds_000127
71. Hms2089k21
72. Hms3742e07
73. Hms3742g13
74. Bcp12188
75. Tox21_110239
76. Hy-17037a
77. Pdsp1_000965
78. Pdsp2_000949
79. Zinc19632927
80. Akos015969751
81. Tox21_110239_1
82. Ccg-205042
83. Db00670
84. Sdccgsbi-0050935.p004
85. Idi1_000127
86. Ncgc00015836-01
87. Ncgc00015836-02
88. Ncgc00015836-03
89. Ncgc00015836-04
90. Ncgc00015836-05
91. Ncgc00015836-06
92. Ncgc00015836-07
93. Ncgc00015836-09
94. Ncgc00015836-10
95. Ncgc00015836-12
96. Ncgc00015836-19
97. Ncgc00024297-02
98. Ncgc00024297-04
99. Ncgc00024297-05
100. Sbi-0050935.p003
101. Cas-29868-97-1
102. Ab00053603
103. Cs-0013749
104. Ft-0600051
105. Ft-0673943
106. 97p617
107. C07508
108. D08389
109. Ab00053603-08
110. Ab00053603_09
111. L000485
112. Q419550
113. Brd-k89375097-300-05-4
114. Brd-k89375097-300-06-2
115. 11-[2-(4-methylpiperazino)acetyl]-5h-pyrido[2,3-b][1,4]benzodiazepin-6-one;hydrochloride
116. 11-[(4-methyl-1-piperazinyl)acetyl]-5,11-dihydro-6h-pyrido[2,3-b][1,4]benzodiazepin-6-one #
117. 11-[2-(4-methyl-1-piperazinyl)-1-oxoethyl]-5h-pyrido[2,3-b][1,4]benzodiazepin-6-one;hydrochloride
118. 11-[2-(4-methyl-piperazin-1-yl)-acetyl]-5,11-dihydro-benzo[e]pyrido[3,2-b][1,4]diazepin-6-one
119. 11-[2-(4-methylpiperazin-1-yl)-acetyl]-5,11-dihydro-6h-pyrido[2,3-b][1,4]benzodiazepin-6-one
120. 11-[2-(4-methylpiperazin-1-yl)acetyl]-5h-pyrido[2,3-b][1,4]benzodiazepin-6-one;hydrochloride
121. 11-[2-(4-methylpiperazin-1-yl)ethanoyl]-5h-pyrido[2,3-b][1,4]benzodiazepin-6-one;hydrochloride
122. 2-[2-(4-methylpiperazin-1-yl)acetyl]-2,4,9-triazatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3,5,7,12,14-hexaen-10-one
| Molecular Weight | 351.4 g/mol |
|---|---|
| Molecular Formula | C19H21N5O2 |
| XLogP3 | 0.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 2 |
| Exact Mass | 351.16952493 g/mol |
| Monoisotopic Mass | 351.16952493 g/mol |
| Topological Polar Surface Area | 68.8 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 534 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of peptic ulcer, gastric ulcer, and duodenal ulcer.
Pirenzepine belongs to a group of medications called antispasmodics/anticholinergics. These medications are used to relieve cramps or spasms of the stomach, intestines, and bladder. Pirenzepine is used to treat duodenal or stomach ulcers or intestine problems. It can be used together with antacids or other medicine in the treatment of peptic ulcer. It may also be used to prevent nausea, vomiting, and motion sickness.
Anti-Ulcer Agents
Various agents with different action mechanisms used to treat or ameliorate PEPTIC ULCER or irritation of the gastrointestinal tract. This has included ANTIBIOTICS to treat HELICOBACTER INFECTIONS; HISTAMINE H2 ANTAGONISTS to reduce GASTRIC ACID secretion; and ANTACIDS for symptomatic relief. (See all compounds classified as Anti-Ulcer Agents.)
Muscarinic Antagonists
Drugs that bind to but do not activate MUSCARINIC RECEPTORS, thereby blocking the actions of endogenous ACETYLCHOLINE or exogenous agonists. Muscarinic antagonists have widespread effects including actions on the iris and ciliary muscle of the eye, the heart and blood vessels, secretions of the respiratory tract, GI system, and salivary glands, GI motility, urinary bladder tone, and the central nervous system. (See all compounds classified as Muscarinic Antagonists.)
A - Alimentary tract and metabolism
A02 - Drugs for acid related disorders
A02B - Drugs for peptic ulcer and gastro-oesophageal reflux disease (gord)
A02BX - Other drugs for peptic ulcer and gastro-oesophageal reflux disease (gord)
A02BX03 - Pirenzepine
Pirenzepine is a muscarinic receptor antagonist and binds to the muscarinic acetylcholine receptor. The muscarinic acetylcholine receptor mediates various cellular responses, including inhibition of adenylate cyclase, breakdown of phosphoinositides and modulation of potassium channels through the action of G proteins.
