

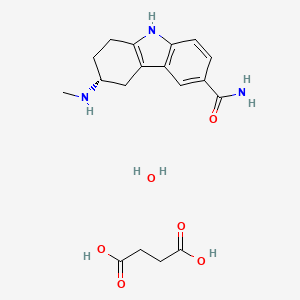

1. (+)-(r)-5,6,7,8-tetrahydro-6-(methylamino)carbazole-3-carboxamide Succinate (1:1), Monohydrate
2. 3-methylamino-6-carboxamido-1,2,3,4-tetrahydrocarbazole
3. Allegro
4. Frova
5. Frovatriptan
6. Frovelan
7. Sb 209509
8. Vml-251
9. Vml251
1. 158930-17-7
2. Frovatriptan Succinate Hydrate
3. Frovatriptan Succinate Monohydrate
4. Frovatriptan Succinate [usan]
5. Frovelan
6. Migard
7. Vml 251
8. Sb 209509-ax
9. D28j6w18hy
10. Sb 209509 Ax
11. Nsc-760422
12. Ncgc00183880-01
13. Butanedioic Acid;(6r)-6-(methylamino)-6,7,8,9-tetrahydro-5h-carbazole-3-carboxamide;hydrate
14. (+)-(r)-2,3,4,9-tetrahydro-3-(methylamino)-1h-carbazole-6-carboxamide Butanedioate (1:1), Monohydrate
15. (+)-(r)-5,6,7,8-tetrahydro-6-(methylamino)carbazole-3-carboxamide Succinate (1:1), Monohydrate
16. Butanedioic Acid, Compd. With (r)-2,3,4,9-tetrahydro-3-(methylamino)-1h-carbazole-6-carboxamide (1:1), Monohydrate
17. Butanedioic Acid, Compd. With (r)-2,3,4,9-tetrahydro-3-(methylamino)-1h-carbazole-6-carboxamide, Hydrate (1:1:1)
18. Frovatriptan Succinate (usan)
19. Frovatriptan (succinate Hydrate)
20. (r)-3-(methylamino)-2,3,4,9-tetrahydro-1h-carbazole-6-carboxamide Succinate Hydrate
21. Butanedioic Acid,(6r)-6-methylamino-6,7,8,9-tetrahydro-5h-carbazole-3-carboxamide;hydrate
22. Unii-d28j6w18hy
23. Frova (tn)
24. Dsstox_cid_28982
25. Dsstox_rid_83247
26. Dsstox_gsid_49056
27. Schembl2321442
28. Chembl2138684
29. Dtxsid2049056
30. Hy-b1658a
31. Tox21_113606
32. Akos030241886
33. Frovatriptan Succinate [mart.]
34. Frovatriptan Succinate [usp-rs]
35. Nsc 760422
36. 1h-carbazole-6-carboxamide, 2,3,4,9-tetrahydro-3-(methylamino)-, (r)-, Butanedioate (1:1), Monohydrate
37. Frovatriptan Succinate [orange Book]
38. Cas-158930-17-7
39. Cs-0021309
40. D04264
41. Frovatriptan Succinate Monohydrate [mi]
42. Frovatriptan Succinate Monohydrate [who-dd]
43. Frovatriptan Succinate Monohydrate, >=97% (hplc)
44. J-009548
45. Q27276007
46. Frovatriptan Succinate, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 379.4 g/mol |
|---|---|
| Molecular Formula | C18H25N3O6 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 5 |
| Exact Mass | 379.17433553 g/mol |
| Monoisotopic Mass | 379.17433553 g/mol |
| Topological Polar Surface Area | 147 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 426 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
Serotonin Receptor Agonists
Endogenous compounds and drugs that bind to and activate SEROTONIN RECEPTORS. Many serotonin receptor agonists are used as ANTIDEPRESSANTS; ANXIOLYTICS; and in the treatment of MIGRAINE DISORDERS. (See all compounds classified as Serotonin Receptor Agonists.)
