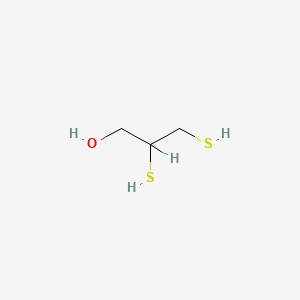

1. 2,3 Dimercaptopropanol
2. 2,3 Dithiopropan 1 O1
3. 2,3-dimercaptopropanol
4. 2,3-dimercaptopropanol, Cadmium
5. 2,3-dithiopropan-1-o1
6. Anti-lewisite Agent, British
7. Anti-lewisite, British
8. B.a.l.
9. Bal In Oil
10. British Anti Lewisite
11. British Anti Lewisite Agent
12. British Anti-lewisite
13. British Anti-lewisite Agent
14. Cadmium 2,3 Dimercaptopropanol
15. Cadmium 2,3-dimercaptopropanol
16. Dicaptol
17. In Oil, Bal
18. Oil, Bal In
1. 2,3-dimercapto-1-propanol
2. 59-52-9
3. 2,3-dimercaptopropanol
4. Dithioglycerine
5. Dicaptol
6. Sulfactin
7. 2,3-dithiopropanol
8. Dimercaptopropanol
9. 1,2-dithioglycerol
10. Dimersol
11. Antoxol
12. Panobal
13. British Anti-lewisite
14. British Antilewisite
15. Dimercaprolum
16. Dithioglycerol
17. 1-propanol, 2,3-dimercapto-
18. 1,2-dimercapto-3-propanol
19. Bal In Oil
20. 3-hydroxy-1,2-propanedithiol
21. 2,3-bis(sulfanyl)propan-1-ol
22. Dimercaprol Propanol
23. 2,3-dimercaptol-1-propanol
24. Bal
25. Usaf Me-1
26. 2,3-dimercaptopropan-1-ol
27. Alpha,beta-dithioglycerol
28. Dimerkaprol
29. 2,3-mercaptopropanol
30. Glycerol, 1,2-dithio-
31. 1-propanol, 2,3-dimercapto
32. 2,3-mercaptopropan-1-ol
33. Nsc 4646
34. 2,3-disulfanylpropan-1-ol
35. Nsc 39515
36. Chebi:64198
37. Nsc-4646
38. 2,3-dithiopropan-1-ol
39. Nsc-39515
40. Dimercaptol
41. 2,3,-dimercapto-1-propanol
42. 2,3-dimercapto-propan-1-ol
43. .alpha.,.beta.-dithioglycerol
44. 0cpp32s55x
45. Nsc4646
46. Glycerol,2-dithio-
47. 2,3-dimercapto-1-propanol; Dithioglycerol
48. Wln: Sh1ysh1q
49. 1-propanol,3-dimercapto-
50. Sulfactin (van)
51. Dimerkaprol [czech]
52. Dimercaprolo [dcit]
53. Dithioglycerol (van)
54. Dimercaprolo
55. Dimercaptopropanol (van)
56. Dmp (van)
57. Dimercaprolum [inn-latin]
58. 2,3-dimercapro
59. Nsc16865
60. Ccris 3616
61. Hsdb 4004
62. Einecs 200-433-7
63. Brn 1732058
64. Dithiopropanol
65. Unii-0cpp32s55x
66. Ai3-61820
67. Anti-lewisite
68. Dimercaprol [usp:inn:ban:jan]
69. Mfcd00004864
70. Spectrum_001965
71. 2, 3-dimercaptopropanol
72. Dimercaprol [mi]
73. Spectrum2_001218
74. Spectrum4_001275
75. Spectrum5_001622
76. Dimercaprol [inn]
77. Dimercaprol [jan]
78. Dimercaprol [hsdb]
79. Dimercaprol [vandf]
80. Bal (tn)
81. Chembl1597
82. Dimercaprol [mart.]
83. Dsstox_cid_20461
84. Dsstox_rid_79496
85. Dsstox_gsid_40461
86. Schembl15969
87. Dimercaprol [who-dd]
88. Dimercaprol [who-ip]
89. Kbiogr_001890
90. Kbioss_002524
91. 2,3 -dimercapto-1-propanol
92. 4-01-00-02770 (beilstein Handbook Reference)
93. Divk1c_000997
94. Spbio_001036
95. 2,3-disulfanyl-1-propanol #
96. Dimercaprol (jp17/usp/inn)
97. Dtxsid5040461
98. Hms503g15
99. Kbio1_000997
100. Kbio2_002516
101. Kbio2_005084
102. Kbio2_007652
103. Dimercaprol [orange Book]
104. Ninds_000997
105. Dimercaprol [ep Monograph]
106. Pharmakon1600-01500252
107. Dimercaprol [usp Monograph]
108. Bcp18145
109. Hy-b1285
110. Nsc39515
111. Tox21_110014
112. Bdbm50103608
113. Ccg-39156
114. Dimercaprolum [who-ip Latin]
115. Nsc760094
116. S4446
117. Akos015895110
118. Db06782
119. Nsc-760094
120. Cas-59-52-9
121. Idi1_000997
122. Ncgc00013216-01
123. Ncgc00344527-01
124. Ls-12962
125. Nci60_001345
126. Sbi-0051351.p002
127. Db-053404
128. Cs-0013059
129. D0621
130. Ft-0625021
131. C02924
132. D00167
133. D89691
134. Ab00051971_02
135. 004d864
136. 2,3-dimercapto-1-propanol, >=98% (iodometric)
137. Q413968
138. Sr-05000001839
139. Sr-05000001839-1
140. W-105317
| Molecular Weight | 124.23 g/mol |
|---|---|
| Molecular Formula | C3H8OS2 |
| XLogP3 | 0.2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Exact Mass | 124.00165722 g/mol |
| Monoisotopic Mass | 124.00165722 g/mol |
| Topological Polar Surface Area | 22.2 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 32 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Bal |
| PubMed Health | Balsalazide (By mouth) |
| Drug Classes | Gastrointestinal Agent |
| Drug Label | Dimercaprol Injection USP is a colorless or almost colorless liquid chelating agent having a disagreeable, mercaptan-like odor. Each 1 mL sterile BAL in Oil (Dimercaprol Injection USP) contains: 100 mg Dimercaprol in 200 mg Benzyl Benzoate and 700 mg... |
| Active Ingredient | Dimercaprol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 10% |
| Market Status | Prescription |
| Company | Akorn |
| 2 of 2 | |
|---|---|
| Drug Name | Bal |
| PubMed Health | Balsalazide (By mouth) |
| Drug Classes | Gastrointestinal Agent |
| Drug Label | Dimercaprol Injection USP is a colorless or almost colorless liquid chelating agent having a disagreeable, mercaptan-like odor. Each 1 mL sterile BAL in Oil (Dimercaprol Injection USP) contains: 100 mg Dimercaprol in 200 mg Benzyl Benzoate and 700 mg... |
| Active Ingredient | Dimercaprol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 10% |
| Market Status | Prescription |
| Company | Akorn |
Antidotes; Chelating Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Dimercaprol is the antidote of choice in the treatment of acute arsenic (except arsine), mercury, or gold poisoning resulting from ingestion of salts of these metals or following overdosage of therapeutic agents containing these metals. Dimercaprol administration should be accompanied by appropriate supportive measures and is most effective when administered early in the course of the poisoning. In the treatment of acute poisoning by mercury salts, dimercaprol is most effective if administered within 1-2 hours following ingestion, since extensive mercury-induced renal damage cannot be reversed. Dimercaprol is not effective in the treatment of poisonings resulting from monoalkyl mercury compounds, and the drug is only minimally effective in chronic mercury poisoning. Although dimercaprol is usually of no value in the treatment of hypersensitivity reactions to mercury compounds, mercury-induced acrodynia (pink disease) in infants and children responds to treatment with dimercaprol. Dimercaprol is usually effective in the treatment of chronic poisoning from inorganic or organic arsenicals, but may be of little value if aplastic anemia, hemorrhagic encephalitis, or jaundice has developed. In one patient who experienced protein-loss enteropathy in association with arsenic poisoning, hypoproteinemia and edema improved following dimercaprol therapy. The drug is ineffective in the treatment of poisoning resulting from arsine gas (AsH3). Gold-induced dermatitis and gold-induced thrombocytopenia may improve following dimercaprol therapy. /Use included in US product label/
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010)
Dermatologic or ocular manifestations of arsenic poisoning have been effectively treated with topical dimercaprol ointment or oil solution, respectively. /Use NOT included in US product label/
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010)
Although dimercaprol chelates lead, other agents (e.g., edetate calcium disodium (calcium EDTA), succimer) generally are preferred for the management of most cases of moderate lead poisoning. However, dimercaprol is useful as an adjunct to edetate calcium disodium and concomitant administration of the drugs is preferred, at least initially, in the management of patients with severe lead poisoning (blood lead concentrations exceeding 70 ug/dL) and/or in those with acute lead encephalopathy (which occurs most often in children). Concomitant administration of dimercaprol and edetate calcium disodium increases the rate of excretion of lead, lowers mortality, and may lower the incidence of brain damage as compared with the use of edetate calcium disodium alone; however, such concomitant therapy does not completely eliminate the risk of permanent severe residual brain damage. Since lead encephalopathy occurs only rarely in adults, experience with the use of the combination in these patients is limited; however, use of dimercaprol and edetate calcium disodium has resulted in prompt relief of symptoms in a few adults with lead encephalopathy. Although concomitant therapy with dimercaprol and edetate calcium disodium also has been recommended in symptomatic patients with blood lead concentrations less than 70 ug/dL, the American Academy of Pediatrics currently states that the toxicity of dimercaprol and the current availability of alternative drugs mandate its use only in the most serious cases of lead poisoning (i.e., blood lead concentrations exceeding 70 ug/dL or when symptoms suggestive of encephalopathy are present). Edetate calcium disodium generally is used alone (i.e., without dimercaprol) in asymptomatic patients with blood lead concentrations of 45-70 ug/dL. ... Dimercaprol is not useful in acute poisonings resulting from alkyl lead compounds (e.g., tetraethyl lead). /Use included in US product label/
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010)
For more Therapeutic Uses (Complete) data for Dimercaprol (9 total), please visit the HSDB record page.
Dimercaprol is potentially nephrotoxic. Since the chelate dissociates in acid medium, the urine should be kept alkaline during dimercaprol therapy to protect the kidneys. Dimercaprol should be used with caution and/or the dosage reduced in patients with oliguria. If acute renal failure develops during therapy, the drug should be discontinued or used very cautiously because serum concentrations of dimercaprol may reach toxic levels.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010)
Adverse effects of dimercaprol are usually mild and transitory and occur in about one-half of patients who receive an IM dose of 5 mg/kg. If the dose of dimercaprol exceeds 5 mg/kg, most patients will experience vomiting, seizures, and stupor or coma which may begin within 30 minutes after injection and usually subside in 1-6 hours. Prophylactic or therapeutic administration of ephedrine or an antihistamine may prevent or relieve many of the mild adverse effects of dimercaprol. The most frequent adverse effect, a rise in systolic and diastolic blood pressure which is dose related and may be accompanied by tachycardia, may appear 15-30 minutes following the injection and blood pressure usually returns to normal within 2 hours. Frequently pain and occasionally sterile abscesses occur at the injection site, particularly if the drug is not administered deep IM.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010)
Other adverse effects that may occur include nausea, vomiting, headache, sweating, and a feeling of constriction (or pain) in the throat, chest, or hands which may be accompanied by anxiety, nervousness or restlessness, and weakness. Muscular aches and pains, muscle spasms, tingling of extremities, and abdominal pain have also been reported.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010)
Dimercaprol has a strong odor and imparts an unpleasant mercaptan-like odor to the patient's breath. The drug may also produce a burning sensation of the lips, mouth, throat, eyes, and penis, and pain in the teeth. Blepharal spasm, conjunctivitis, lacrimation, rhinorrhea, and salivation may also occur. When the drug is applied topically, it produces erythema and edema.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010)
For more Drug Warnings (Complete) data for Dimercaprol (16 total), please visit the HSDB record page.
For the treatment of arsenic, gold and mercury poisoning. Indicated in acute lead poisoning when used concomitantly with edetate calcium disodium (DB00974).
FDA Label
Due to its oily nature, dimercaprol is not absorbed orally and its administration requires a deep intra-muscular injection that is extremely painful and allergenic. It was found to mobilize and relocate lead to the brain, increasing its neurotoxic effects. Despite that fact that dimercaprol increases cadmium excretion, there is an associated increase in kidney cadmium concentration. Because of this, dimercaprol must be avoided in patients with cadmium toxicity.
Chelating Agents
Chemicals that bind to and remove ions from solutions. Many chelating agents function through the formation of COORDINATION COMPLEXES with METALS. (See all compounds classified as Chelating Agents.)
V - Various
V03 - All other therapeutic products
V03A - All other therapeutic products
V03AB - Antidotes
V03AB09 - Dimercaprol
Absorption
After intra-muscular injection.
Route of Elimination
Urine.
Because it is a lipophilic drug, dimercaprol penetrates rapidly the intracellular spaces. The highest concentrations are found in the liver, kidneys, brain and small intestine. Due to its lipophilic characteristic, the complexes formed with mercury and other metals may be redistributed into sensitive cells in the brain following dimercaprol treatment.
International Programme on Chemical Safety (IPCS); Poisons Information Monograph: Dimercaprol (PIM 193) (1990) Available from, as of June 16, 2010: https://www.inchem.org/documents/pims/pharm/dimercap.htm
It is readily absorbed through the skin after topical application. Percutaneous absorption in rats and humans equals 3 millimol (124 mg)/sq cm per hour.
International Programme on Chemical Safety (IPCS); Poisons Information Monograph: Dimercaprol (PIM 193) (1990) Available from, as of June 16, 2010: https://www.inchem.org/documents/pims/pharm/dimercap.htm
Following absorption, dimercaprol is distributed to all tissues (mainly in the intracellular space) including the brain, with highest concentrations in the liver and kidneys.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010)
Following IM injection of therapeutic doses of dimercaprol, peak blood concentrations are attained in 30-60 minutes. Dimercaprol is slowly absorbed through the skin following topical application.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010)
Dimercaprol is not absorbed orally. It is rapidly absorbed after intramuscular injection and persists for at least 12 hours. Approximately 80% of the dose is absorbed after 1 hours and 90% after 6 hours. Maximal blood concentrations are attained within 1 hour. Hepatic metabolism (by glucuronidation) and excretion are essentially complete within 4 hours. Dimercaprol is the only commonly used chelating agent that readily crosses cellular membranes; as a result, the concentration in certain organs (liver, kidney, small intestine) can be up to five times that in the blood.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 186
... Metabolic degradation and excretion are essentially complete within 4 hours.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill, 2006., p. 1770
Dimercaprol not excreted as dimercaprol-metal complex is quickly metabolized by the liver and excreted as an inactive product in the urine.
Sullivan, J.B. Jr., G.R. Krieger (eds.). Hazardous Materials Toxicology-Clinical Principles of Environmental Health. Baltimore, MD: Williams and Wilkins, 1992., p. 409
The drug has a short half life.
The sulfhydryl groups of dimercaprol form complexes with certain heavy metals thus preventing or reversing the metallic binding of sulfhydryl-containing enzymes. The complex is excreted in the urine.
Dimercaprol is much more effective when given as soon as possible after exposure to the metal because it is more effective in preventing inhibition of sulfhydryl enzymes than in reactivating them. Dimercaprol antagonizes the biological actions of metals that form mercaptides with essential cellular sulfhydryl groups, principally arsenic, gold, and mercury. It also is used in combination with CaNa2EDTA to treat lead poisoning, especially when evidence of lead encephalopathy exists. Intoxication by selenites, which also oxidize sulfhydryl enzymes, is not influenced by dimercaprol
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill, 2006., p. 1770
The pharmacological actions of dimercaprol result from formation of chelation complexes between its sulfhydryl groups and metals. The molecular properties of the dimercaprol-metal chelate have considerable practical significance. With metals such as mercury, gold, and arsenic, the strategy is to attain a stable complex to promote elimination of the met al. Dissociation of the complex and oxidation of dimercaprol can occur in vivo. Furthermore, the sulfur-metal bond may be labile in the acidic tubular urine, which may increase delivery of metal to renal tissue and increase toxicity. The dosage regimen therefore is designed to maintain a concentration of dimercaprol in plasma adequate to favor the continuous formation of the more stable 2:1 (BAL-metal) complex and its rapid excretion. However, because of pronounced and dose-related side effects, excessive plasma concentrations must be avoided. The concentration in plasma therefore must be maintained by repeated fractional dosage until the offending metal can be excreted.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill, 2006., p. 1770
Ca2+ is involved in the regulation of a variety of physiological processes, but a persistent increase in free cytosolic Ca2+ concentrations may contribute to cell injury. Dimercaprol (BAL) is a compound used in the treatment of mercury intoxication, but presents low therapeutic efficacy. The molecular mechanism responsible for the BAL toxicity is poorly known. In the present study, the effect of BAL and inorganic and organic mercury on Ca2+ transport by Ca2+-ATPases located in the sarco/endoplasmic reticulum of fast-skeletal muscle and brain was examined. Ca2+ uptake by brain and fast-skeletal muscle microsomes was inhibited in a dose-dependent manner by Hg2+. The calculated IC50 for Ca2+ uptake inhibition by HgCl2 was 1.05+/-0.09 microM (n = 8) for brain and 0.72+/-0.06 microM (n = 9) for muscle. The difference was significant at p < 0.01 (data expressed as mean +/- SD). At a low concentration (1 microM), 2,3-dimer-captopropanol had no effect on Ca2+ uptake by brain or muscle vesicles and did not abolish the inhibition caused by Hg2+. A high concentration of BAL (1 mM) nearly abolished the inhibition caused by 1.75 microM HgCl2 or 6 microM CH3HgCl in skeletal muscle. Surprisingly, at intermediate concentrations (40-100 microM) BAL partially inhibited Ca2+ transport in brain but had no effect on muscle. Furthermore, ATP hydrolysis by brain or muscle microsomes was not inhibited by BAL. These results suggest that in brain microsomes BAL affects in a different way Ca2+ transport and ATP hydrolysis. The increase in BAL concentration observed after toxic administration of this compound to experimental animals may contribute to deregulate Ca2+ homoeostasis and, consequently, to the neurotoxicity of BAL.
PMID:11495549 Quinhones EB et al; Neurochem Res 26 (3): 251-6 (2001)