

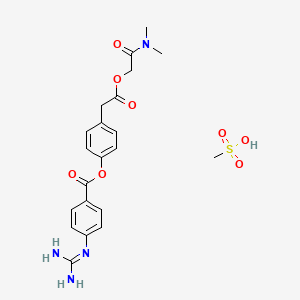

1. Camostat
2. Camostat Mesilate
3. Camostate
4. Camostate-mesilate
5. Foipan
6. Foy 305
7. Foy S 980
8. Foy-305
9. Foypan
10. N,n-dimethylcarbamoylmethyl-4-(4-guanidinobenzoyloxy)phenylacetate Methanesulfonate
11. P-guanidinobenzoic Acid, Ester With (p-hydroxyphenyl)acetic Acid, Ester With N,n-dimethylglycolamide
1. Camostat Mesilate
2. 59721-29-8
3. Camostat (mesylate)
4. Camostat Methanesulfonate
5. Foipan
6. Camostate
7. Mfcd00941410
8. Foy305
9. Camostat Mesilate [jan]
10. Camostat Mesilate;foy305;foy-s980
11. Dsstox_cid_238
12. 4-(2-(2-(dimethylamino)-2-oxoethoxy)-2-oxoethyl)phenyl 4-guanidinobenzoate Methanesulfonate
13. Dsstox_rid_75452
14. Dsstox_gsid_20238
15. Chembl85164
16. 2-(dimethylamino)-2-oxoethyl 4-(4-guanidinobenzoyloxy)phenylacetate Methanesulphonate
17. Methanesulfonic Acid 4-{2-[(dimethylcarbamoyl)methoxy]-2-oxoethyl}phenyl 4-carbamimidamidobenzoate
18. 451m50a1eq
19. Cas-59721-29-8
20. Ncgc00167526-01
21. Foy-305
22. Foipan (tn)
23. Camostat Mesylate- Bio-x
24. Camostat Mesilate (jp17)
25. Mls006010697
26. Schembl871583
27. Foy-s980
28. Camostat Mesilate (foy-305)
29. Dtxsid0020238
30. Chebi:31347
31. Amy8832
32. Camostat Mesilate [mart.]
33. Hms3651d04
34. Camostat Mesilate [who-dd]
35. Bcp06708
36. Ex-a4154
37. Tox21_112523
38. Camostat Mesylate, >=98% (hplc)
39. S2874
40. Camostat Methanesulfonate [mi]
41. Akos015966692
42. Akos026750463
43. Tox21_112523_1
44. Ac-8540
45. Ccg-269652
46. Hs-0060
47. Ncgc00167526-02
48. 4-(2-(2-(dimethylamino)-2-oxoethoxy)-2-oxoethyl)phenyl 4-((diaminomethylene)amino)benzoate Methanesulfonate
49. 4-[[4-[(aminoiminomethyl)amino]benzoyl]oxy]benzeneacetic Acid 2-(dimethylamino)-2-oxoethyl Ester Methanesulfonate
50. Bc164270
51. Hy-13512
52. Smr002530343
53. Sy060177
54. Db-072684
55. B2082
56. Ft-0648873
57. Sw219517-1
58. D01766
59. F20649
60. 721c298
61. A832436
62. Q-200778
63. Q23731028
64. 4-(2-(2-(dimethylamino)-2-oxoethoxy)-2-oxoethyl)phenyl4-guanidinobenzoatemethanesulfonate
65. 4-(2-{[2-(dimethylamino)-2-oxoethyl]oxy}-2-oxoethyl)phenyl 4-{[amino(imino)methyl]amino}benzoate Methanesulfonate
66. 4-{2-[(dimethylcarbamoyl)methoxy]-2-oxoethyl}phenyl 4-[(diaminomethylidene)amino]benzoate; Methanesulfonic Acid
| Molecular Weight | 494.5 g/mol |
|---|---|
| Molecular Formula | C21H26N4O8S |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 9 |
| Exact Mass | 494.14713497 g/mol |
| Monoisotopic Mass | 494.14713497 g/mol |
| Topological Polar Surface Area | 200 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 695 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Trypsin Inhibitors
Serine proteinase inhibitors which inhibit trypsin. They may be endogenous or exogenous compounds. (See all compounds classified as Trypsin Inhibitors.)
Protease Inhibitors
Compounds which inhibit or antagonize biosynthesis or actions of proteases (ENDOPEPTIDASES). (See all compounds classified as Protease Inhibitors.)
