

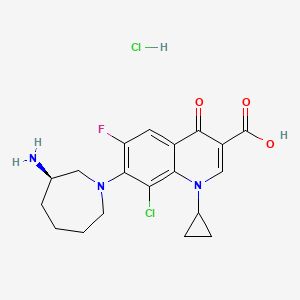

1. 7-((3r)-aminohexahydro-1h-azepin-1-yl)-8-chloro-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic Acid
2. 7-(3-aminohexahydro-1h-azepin-1-yl)-8-chloro-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic Acid
3. Besifloxacin
4. Besivance
5. Bol-303224-a
1. Besifloxacin Hcl
2. 405165-61-9
3. Besivance
4. Besifloxacin (hydrochloride)
5. Bol-303224-a
6. Besifloxacin Hydrochloride [usan]
7. Ss734
8. Besifloxacin Hcl (besivance)
9. Besifloxacin (as Hydrochloride)
10. Ss-734
11. 7506a6j57t
12. 405165-61-9 (hcl)
13. Unii-7506a6j57t
14. Besifloxacin Hydrochloride (usan)
15. (+)-7-((3r)-3-aminohexahydro-1h-azepin-1-yl)-8-chloro-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid Hydrochloride
16. (+)-7-[(3r)-3-aminohexahydro-1h-azepin-1-yl]-8-chloro-1- Cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid Hydrochloride.
17. (r)-7-(3-aminoazepan-1-yl)-8-chloro-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid Hydrochloride
18. 3-quinolinecarboxylic Acid, 7-((3r)-3-aminohexahydro-1h-azepin-1-yl)-8-chloro-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-, Monohydrochloride
19. 7-[(3r)-3-aminoazepan-1-yl]-8-chloro-1-cyclopropyl-6-fluoro-4-oxoquinoline-3-carboxylic Acid;hydrochloride
20. Bol 303224a
21. Besivance (tn)
22. Besivance Hydrochloride
23. 7-[(3r)-3-aminohexahydro-1h-azepin-1-yl]-8-chloro-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic Acid Hydrochloride
24. Schembl291598
25. Chembl1201761
26. Dtxsid20193529
27. Besifloxacin Hydrochloride- Bio-x
28. Amy18155
29. Ex-a1339
30. Mfcd14636632
31. S3055
32. Akos025401618
33. Besifloxacin Hydrochloride [mi]
34. Ccg-221259
35. Cs-0713
36. Ac-23367
37. Bb164241
38. Besifloxacin Hydrochloride [mart.]
39. Besifloxacin Hydrochloride [vandf]
40. Hy-17028
41. Besifloxacin Hydrochloride [who-dd]
42. Besifloxacin Hydrochloride [orange Book]
43. C75052
44. D08872
45. 165b619
46. A848253
47. J-519840
48. Q27266315
49. Besifloxacin Hydrochloride 100 Microg/ml In Acetonitrile:water
50. (r)-7-(3-aminohexahydro-1h-azepin-1-yl)-8-chloro-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic Acid Hydrochloride
| Molecular Weight | 430.3 g/mol |
|---|---|
| Molecular Formula | C19H22Cl2FN3O3 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 3 |
| Exact Mass | 429.1022251 g/mol |
| Monoisotopic Mass | 429.1022251 g/mol |
| Topological Polar Surface Area | 86.9 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 656 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Topoisomerase II Inhibitors
Compounds that inhibit the activity of DNA TOPOISOMERASE II. Included in this category are a variety of ANTINEOPLASTIC AGENTS which target the eukaryotic form of topoisomerase II and ANTIBACTERIAL AGENTS which target the prokaryotic form of topoisomerase II. (See all compounds classified as Topoisomerase II Inhibitors.)
