
1. Tessalon
1. 104-31-4
2. Benzononatine
3. Tessalon
4. Benzononantin
5. Exangit
6. Ventussin
7. Benzonatatum
8. Tessalon-ciba
9. Ventussin-loz
10. Benzonatato
11. Tessalin
12. Zonatuss
13. Benzonatatum [inn-latin]
14. Benzonatato [inn-spanish]
15. Km65
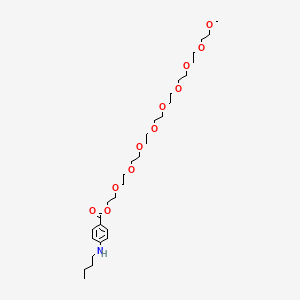
16. 2,5,8,11,14,17,20,23,26-nonaoxaoctacosan-28-yl P-(butylamino)benzoate
17. P-(n)-butylaminobenzoesaeure-(nonaaethylenglykol-monomethylaether)-ester
18. 2,5,8,11,14,17,20,23,26-nonaoxaoctacosan-28-yl 4-(butylamino)benzoate
19. Benzoic Acid, 4-(butylamino)-, 3,6,9,12,15,18,21,24,27-nonaoxaoctacos-1-yl Ester
20. Chebi:3032
21. Nonaethyleneglycol Monomethyl Ether P-n-butylaminobenzoate
22. Tesalon
23. 4-(butylamino)benzoic Acid 3,6,9,12,15,18,21,24,27-nonaoxaoctacos-1-yl Ester
24. Benzoic Acid, 4-(butylamino)-, 2,5,8,11,14,17,20,23,26-nonaoxaoctacos-28-yl Ester
25. Nsc-760128
26. 2-[2-[2-[2-[2-[2-[2-[2-(2-methoxyethoxy)ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethyl 4-butylaminobenzoate
27. Benzoic Acid, P-(butylamino)-, 2-(2-(2-(2-(2-(2-(2-(2-(2-methoxyethoxy)ethoxy)ethoxy)ethoxy)ethoxy)ethoxy)ethoxy)ethoxy)ethyl Ester
28. Ncgc00016362-01
29. Cas-104-31-4
30. P-butylaminobenzoic Acid Omega-o-methylnonaethyleneglycol Ester
31. 3,6,9,12,15,18,21,24,27-nonaoxaoctacosyl 4-butylaminobenzoate
32. Dsstox_cid_2655
33. Dsstox_rid_76676
34. Dsstox_gsid_22655
35. 3,6,9,12,15,18,21,24,27-nonaoxaoctacosyl-4-butylaminobenzoate;3,6,9,12,15,18,21,24,27-nonaoxaoctacosyl-4-butylaminobenzoate
36. Tessalon Perles (tn)
37. Benzonatate (usp/inn)
38. Einecs 203-194-7
39. Polyethyleneglycol-p-n-butylaminobenzoate Methyl Ester
40. Benzonatate [usp:inn:ban]
41. Hsdb 7933
42. P-(n)-butylaminobenzoesaeure-(nonaaethylenglykol-monomethylaether)-ester [german]
43. Prestwick0_000012
44. Prestwick1_000012
45. Prestwick2_000012
46. Prestwick3_000012
47. Schembl28366
48. Bspbio_000043
49. 2-[2-[2-[2-[2-[2-[2-[2-(2-methoxyethoxy)ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethyl 4-(butylamino)benzoate
50. Mls002154171
51. Spbio_001964
52. Bpbio1_000049
53. Gtpl7611
54. Chembl1374379
55. Dtxsid9022655
56. Hms1568c05
57. Hms2095c05
58. Hms2230m19
59. Hms3373i11
60. Hms3712c05
61. Km-65
62. Pharmakon1600-01503864
63. Hy-b1551
64. Zinc3830276
65. Tox21_110398
66. Nsc760128
67. 3,6,9,12,15,18,21,24,27-nonaoxaoctacos-1-yl 4-(butylamino)benzoate
68. Akos015891366
69. Tox21_110398_1
70. Ccg-213706
71. Db00868
72. Nsc 760128
73. Ncgc00016362-02
74. Ncgc00016362-03
75. Ncgc00016362-05
76. Smr001233469
77. Ab00513795
78. Cs-0013413
79. Ft-0622718
80. D00242
81. Ab00513795_06
82. Sr-01000841197
83. J-001145
84. Q2070778
85. Sr-01000841197-2
86. Nonaethyleneglycol-p-n-butylaminobenzoate Methyl Ester
87. 3,6,9,12,15,18,21,24,27-nonaoxaoctacos-1-yl 4-(butylamino)benzoate #
| Molecular Weight | 603.7 g/mol |
|---|---|
| Molecular Formula | C30H53NO11 |
| XLogP3 | 2.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 33 |
| Exact Mass | 603.36186151 g/mol |
| Monoisotopic Mass | 603.36186151 g/mol |
| Topological Polar Surface Area | 121 Ų |
| Heavy Atom Count | 42 |
| Formal Charge | 0 |
| Complexity | 565 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Benzonatate |
| PubMed Health | Benzonatate (By mouth) |
| Drug Classes | Antitussive |
| Drug Label | Benzonatate, a non-narcotic oral antitussive agent, is 2, 5, 8, 11, 14, 17, 20, 23, 26-nonaoxaoctacosan-28-yl p-(butylamino) benzoate; with a molecular weight of 603.7.Each soft gelatin capsule, for oral administration, contains 100 mg or 200 mg of b... |
| Active Ingredient | Benzonatate |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 200mg; 100mg; 150mg |
| Market Status | Prescription |
| Company | Thepharmanetwork; Orit Labs; Sun Pharm Inds; Zydus Pharms Usa; The Pharma Network; Banner Pharmacaps; Mikart |
| 2 of 4 | |
|---|---|
| Drug Name | Tessalon |
| PubMed Health | Benzonatate (By mouth) |
| Drug Classes | Antitussive |
| Drug Label | TESSALON, a non-narcotic oral antitussive agent, is 2, 5, 8, 11, 14, 17, 20, 23, 26-nonaoxaoctacosan-28-yl p-(butylamino) benzoate; with a molecular weight of 603.7.Each TESSALON Perle contains:Benzonatate, USP 100 mgTESSALON Perles (capsules) also c... |
| Active Ingredient | Benzonatate |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 200mg; 100mg |
| Market Status | Prescription |
| Company | Pfizer |
| 3 of 4 | |
|---|---|
| Drug Name | Benzonatate |
| PubMed Health | Benzonatate (By mouth) |
| Drug Classes | Antitussive |
| Drug Label | Benzonatate, a non-narcotic oral antitussive agent, is 2, 5, 8, 11, 14, 17, 20, 23, 26-nonaoxaoctacosan-28-yl p-(butylamino) benzoate; with a molecular weight of 603.7.Each soft gelatin capsule, for oral administration, contains 100 mg or 200 mg of b... |
| Active Ingredient | Benzonatate |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 200mg; 100mg; 150mg |
| Market Status | Prescription |
| Company | Thepharmanetwork; Orit Labs; Sun Pharm Inds; Zydus Pharms Usa; The Pharma Network; Banner Pharmacaps; Mikart |
| 4 of 4 | |
|---|---|
| Drug Name | Tessalon |
| PubMed Health | Benzonatate (By mouth) |
| Drug Classes | Antitussive |
| Drug Label | TESSALON, a non-narcotic oral antitussive agent, is 2, 5, 8, 11, 14, 17, 20, 23, 26-nonaoxaoctacosan-28-yl p-(butylamino) benzoate; with a molecular weight of 603.7.Each TESSALON Perle contains:Benzonatate, USP 100 mgTESSALON Perles (capsules) also c... |
| Active Ingredient | Benzonatate |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 200mg; 100mg |
| Market Status | Prescription |
| Company | Pfizer |
Antitussive Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 2011)
Tessalon is indicated for the symptomatic relief of cough. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for TESSALON (benzonatate) capsule (February 2011). Available from, as of July 7, 2011: https://dailymed.nlm.nih.gov/dailymed/search.cfm?startswith=benzonatate
Accidental ingestion of Tessalon resulting in death has been reported in children below age 10. Signs and symptoms of overdose have been reported within 15-20 minutes and death has been reported within one hour of ingestion. If accidental ingestion occurs, seek medical attention immediately
US Natl Inst Health; DailyMed. Current Medication Information for TESSALON (benzonatate) capsule (February 2011). Available from, as of July 7, 2011: https://dailymed.nlm.nih.gov/dailymed/search.cfm?startswith=benzonatate
Safety and effectiveness in children below the age of 10 have not been established. Accidental ingestion resulting in death has been reported in children below age 10. Keep out of reach of children.
US Natl Inst Health; DailyMed. Current Medication Information for TESSALON (benzonatate) capsule (February 2011). Available from, as of July 7, 2011: https://dailymed.nlm.nih.gov/dailymed/search.cfm?startswith=benzonatate
Severe hypersensitivity reactions (including bronchospasm, laryngospasm and cardiovascular collapse) have been reported which are possibly related to local anesthesia from sucking or chewing the capsule instead of swallowing it. Severe reactions have required intervention with vasopressor agents and supportive measures.
US Natl Inst Health; DailyMed. Current Medication Information for TESSALON (benzonatate) capsule (February 2011). Available from, as of July 7, 2011: https://dailymed.nlm.nih.gov/dailymed/search.cfm?startswith=benzonatate
Benzonatate is chemically related to anesthetic agents of the para-amino-benzoic acid class (e.g. procaine; tetracaine) and has been associated with adverse CNS effects possibly related to a prior sensitivity to related agents or interaction with concomitant medication.
US Natl Inst Health; DailyMed. Current Medication Information for TESSALON (benzonatate) capsule (February 2011). Available from, as of July 7, 2011: https://dailymed.nlm.nih.gov/dailymed/search.cfm?startswith=benzonatate
For more Drug Warnings (Complete) data for Benzonatate (8 total), please visit the HSDB record page.
Benzonatate is indicated for the symptomatic relief of cough.
Benzonatate suppresses cough associated with both acute and chronic respiratory conditions. Its works by desensitizing the pulmonary stretch receptors involved in the cough reflex. There are limited clinical trials of benzonatate; however, earlier studies demonstrated inhibition of experimentally-induced cough and subjectively-measured pathological cough by benzonatate. Benzonatate has no inhibitory effects on the respiratory center in recommended dosage. Its onset of action is within 15 to 20 minutes following administration and its duration of effect is about 3 to 8 hours.
Antitussive Agents
Agents that suppress cough. They act centrally on the medullary cough center. EXPECTORANTS, also used in the treatment of cough, act locally. (See all compounds classified as Antitussive Agents.)
R - Respiratory system
R05 - Cough and cold preparations
R05D - Cough suppressants, excl. combinations with expectorants
R05DB - Other cough suppressants
R05DB01 - Benzonatate
Absorption
Following oral administration, benzonatate enters the systemic circulation via gastrointestinal absorption. The Cmax of benzonatate following oral administration of 100 mg in healthy Chinese volunteers was 1063 460 ng/mL.
Route of Elimination
There is limited information on the route of elimination of benzonatate.
Volume of Distribution
There is limited information on the volume of distribution of benzonatate.
Clearance
There is limited information on the clearance of benzonatate.
Benzonatate is hydrolyzed to the major metabolite 4-(butylamino)benzoic acid (BABA) by plasma butyrylcholinesterase (BChE).
The half life of benzonatate following oral administration of 100 mg in healthy Chinese volunteers was 1.01 0.41 h.
Benzonatate is a local anesthetic drug that acts peripherally by anesthetizing and reducing the activity of vagal stretch receptors or nerve fibres located in the respiratory passages, lungs, and pleura. Once the stretch receptors are stimulated, they send impulses to the cough centre located in the medulla via an afferent pathway consisting of sensory nerve fibres or the vagus nerve. The efferent signal is then generated that sends impulses to the expiratory muscles to produce a cough. Anesthetizing these receptors by benzonatate results in the inhibition of the cough reflex activity and cough production. Benzonatate also inhibits the transmission of impulses of the cough reflex in the vagal nuclei of the medulla. There are several proposed mechanisms of benzonatate; it is also a potent voltage-gated sodium channel inhibitor.
Benzonatate apparently inhibits cough production by anesthetizing stretch receptors of vagal afferent fibers in the bronchi, alveoli, and pleura that mediate the cough reflex; the drug also suppresses transmission of the cough reflex at the level of the medulla where the afferent impulse is transmitted to the motor nerves. The relationship between local anesthetic action and peripheral action on sensory nerve endings is not clear.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011