

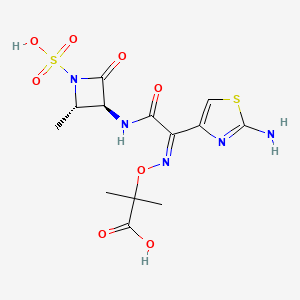

1. Az Threonam
2. Az-threonam
3. Azactam
4. Azthreonam
5. Sq 26,776
6. Sq-26,776
7. Sq26,776
8. Urobactam
1. Azactam
2. 78110-38-0
3. Primbactam
4. Azthreonam
5. Nebactam
6. Azonam
7. Aztreon
8. Rel-aztreonam
9. Sq 26776
10. Sq-26776
11. Nsc646279
12. Chembl158
13. Nsc-646279
14. 149496-40-2
15. Aztreonam E-isomer
16. Nsc-758913
17. 2-[(z)-[1-(2-amino-1,3-thiazol-4-yl)-2-[[(2s,3s)-2-methyl-4-oxo-1-sulfoazetidin-3-yl]amino]-2-oxoethylidene]amino]oxy-2-methylpropanoic Acid
18. Mfcd00072145
19. G2b4ve5gh8
20. Chebi:161680
21. Squibb 26776
22. Sr-01000841814
23. 2-[[(z)-[1-(2-amino-4-thiazolyl)-2-[[(2s,3s)-2-methyl-4-oxo-1-sulfo-3-azetidinyl]amino]-2-oxoethylidene]amino]oxy]-2-methylpropionic Acid
24. (z)-2-((((2-amino-4-thiazolyl)(((2s,3s)-2-methyl-4-oxo-1-sulfo-3-azetidinyl)carbamoyl)methylene)amino)oxy)-2-methylpropionic Acid
25. Aztreonam (azactam, Cayston)
26. Azetreonam
27. E-aztreonam
28. Prestwick_914
29. Aztreonam [inn]
30. Aztreonam [jan]
31. Aztreonam [mi]
32. Aztreonam [usan]
33. Prestwick2_000185
34. Prestwick3_000185
35. Aztreonam [vandf]
36. Aztreonam [mart.]
37. Aztreonam [usp-rs]
38. Aztreonam [who-dd]
39. Bspbio_000109
40. [2s-[2alpha,3beta(z)]]-2-[[[1-(2-amino-4-thiazolyl)-2-[(2-methyl-4-oxo-1-sulfo-3-azetidinyl)amino]-2-oxoethylidene]amino]oxy]-2-methylpropanoic Acid
41. Mls003915628
42. Mls006011974
43. Aztreonam, Analytical Standard
44. Bidd:gt0765
45. Bpbio1_000121
46. Aztreonam [orange Book]
47. Dtxsid0022640
48. Aztreonam [usp Monograph]
49. Bcpp000356
50. Hms1568f11
51. Hms2090k09
52. Hms2095f11
53. Hms3712f11
54. Hy-b0129
55. Bdbm50240480
56. Zinc12503091
57. Akos015840157
58. Akos015961777
59. Ac-4330
60. Bcp9000372
61. Ccg-220185
62. Cs-1902
63. Ncgc00179656-01
64. 2-({[(1z)-1-(2-amino-1,3-thiazol-4-yl)-2-{[(2s,3s)-2-methyl-4-oxo-1-sulfoazetidin-3-yl]amino}-2-oxoethylidene]amino}oxy)-2-methylpropanoic Acid
65. 2-[(z)-[1-(2-aminothiazol-4-yl)-2-[[(2s,3s)-2-methyl-4-oxo-1-sulfo-azetidin-3-yl]amino]-2-oxo-ethylidene]amino]oxy-2-methyl-propanoic Acid
66. As-13760
67. Smr002204030
68. Smr004703537
69. So 26776
70. S1505
71. 110a380
72. Sr-01000841814-2
73. Sr-01000841814-3
74. Brd-k62607865-001-03-0
75. Q27262730
76. Aztreonam, United States Pharmacopeia (usp) Reference Standard
77. Aztreonam, Pharmaceutical Secondary Standard; Certified Reference Material
78. (2s,3s)-3-({(2z)-2-(2-ammonio-1,3-thiazol-4-yl)-2-[(1-carboxy-1-methylethoxy)imino]ethanoyl}amino)-2-methyl-4-oxoazetidine-1-sulfonate
79. (2s,3s)-3-({(2z)-2-(2-ammonio-1,3-thiazol-4-yl)-2-[(1-carboxy-1-methylethoxy)imino]ethanoyl}amino)-2-methyl-4-oxoazetidine-1-sulfonate(aztreonam)
80. 2-((((e)-1-(2-aminothiazol-4-yl)-2-(((2s,3s)-2-methyl-4-oxo-1-sulfoazetidin-3-yl)amino)-2-oxoethylidene)amino)oxy)-2-methylpropanoic Acid
81. 2-((((z)-1-(2-aminothiazol-4-yl)-2-(((2s,3s)-2-methyl-4-oxo-1-sulfoazetidin-3-yl)amino)-2-oxoethylidene)amino)oxy)-2-methylpropanoic Acid
82. 2-[(z)-[1-(2-amino-1,3-thiazol-4-yl)-2-[[(2s)-2-methyl-4-oxo-1-sulfoazetidin-3-yl]amino]-2-oxoethylidene]amino]oxy-2-methylpropanoic Acid
83. 2-[1-(2-amino-thiazol-4-yl)-1-((2s,3s)-2-methyl-4-oxo-1-sulfo-azetidin-3-ylcarbamoyl)-meth-(z)-ylideneaminooxy]-2-methyl-propionic Acid
84. 2-aminothiazol-4-yl)-2-((2s,3s)-2-methyl-4-oxo-1-sulfoazetidin-3-ylamino)-2-oxoethylideneaminooxy)-2-methylpropanoic Acid
85. 80951-91-3
86. Propanoic Acid, 2-(((1-(2-amino-4-thiazolyl)-2-((2-methyl-4-oxo-1-sulfo-3-azetidinyl)amino)-2-oxoethylidene)amino)oxy)-2-methyl-, (2s-(2.alpha.,3.beta.(z)))-
87. Propanoic Acid, 2-[[(z)-[1-(2-amino-4-thiazolyl)-2-[[(2s,3s)-2-methyl-4-oxo-1-sulfo-3-azetidinyl]amino]-2-oxoethylidene]amino]oxy]-2-methyl-
| Molecular Weight | 435.4 g/mol |
|---|---|
| Molecular Formula | C13H17N5O8S2 |
| XLogP3 | 0.3 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 7 |
| Exact Mass | 435.05185486 g/mol |
| Monoisotopic Mass | 435.05185486 g/mol |
| Topological Polar Surface Area | 238 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 808 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Azactam |
| PubMed Health | Aztreonam (Injection) |
| Drug Classes | Antibiotic |
| Drug Label | AZACTAM (aztreonam injection) contains the active ingredient aztreonam, a monobactam. It was originally isolated from Chromobacterium violaceum. It is a synthetic bactericidal antibiotic.The monobactams, having a unique monocyclic beta-lactam nucle... |
| Active Ingredient | Aztreonam |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 2gm/vial; 1gm/vial |
| Market Status | Prescription |
| Company | Bristol Myers Squibb |
| 2 of 6 | |
|---|---|
| Drug Name | Aztreonam |
| PubMed Health | Aztreonam |
| Drug Classes | Antibiotic |
| Drug Label | AZACTAM (aztreonam injection) contains the active ingredient aztreonam, a monobactam. It was originally isolated from Chromobacterium violaceum. It is a synthetic bactericidal antibiotic.The monobactams, having a unique monocyclic beta-lactam nucle... |
| Active Ingredient | Aztreonam |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 500mg/vial; 2gm/vial; 1gm/vial |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa; Eurohlth Intl |
| 3 of 6 | |
|---|---|
| Drug Name | Cayston |
| PubMed Health | Aztreonam |
| Drug Classes | Antibiotic |
| Active Ingredient | Aztreonam |
| Dosage Form | For solution |
| Route | Inhalation |
| Strength | 75mg/vial |
| Market Status | Prescription |
| Company | Gilead |
| 4 of 6 | |
|---|---|
| Drug Name | Azactam |
| PubMed Health | Aztreonam (Injection) |
| Drug Classes | Antibiotic |
| Drug Label | AZACTAM (aztreonam injection) contains the active ingredient aztreonam, a monobactam. It was originally isolated from Chromobacterium violaceum. It is a synthetic bactericidal antibiotic.The monobactams, having a unique monocyclic beta-lactam nucle... |
| Active Ingredient | Aztreonam |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 2gm/vial; 1gm/vial |
| Market Status | Prescription |
| Company | Bristol Myers Squibb |
| 5 of 6 | |
|---|---|
| Drug Name | Aztreonam |
| PubMed Health | Aztreonam |
| Drug Classes | Antibiotic |
| Drug Label | AZACTAM (aztreonam injection) contains the active ingredient aztreonam, a monobactam. It was originally isolated from Chromobacterium violaceum. It is a synthetic bactericidal antibiotic.The monobactams, having a unique monocyclic beta-lactam nucle... |
| Active Ingredient | Aztreonam |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 500mg/vial; 2gm/vial; 1gm/vial |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa; Eurohlth Intl |
| 6 of 6 | |
|---|---|
| Drug Name | Cayston |
| PubMed Health | Aztreonam |
| Drug Classes | Antibiotic |
| Active Ingredient | Aztreonam |
| Dosage Form | For solution |
| Route | Inhalation |
| Strength | 75mg/vial |
| Market Status | Prescription |
| Company | Gilead |
Cayston is indicated for the suppressive therapy of chronic pulmonary infections due to Pseudomonas aeruginosa in patients with cystic fibrosis (CF) aged 6 years and older.
Consideration should be given to official guidance on the appropriate use of antibacterial agents.
Treatment of Gram-negative endobronchial infection in bronchiectasis patients
Treatment of Pseudomonas aeruginosa pulmonary infection / colonisation in patients with cystic fibrosis
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J01DF01
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01D - Other beta-lactam antibacterials
J01DF - Monobactams
J01DF01 - Aztreonam

