





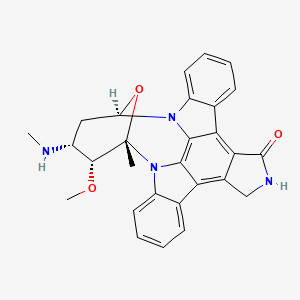

1. 8,12-epoxy-1h,8h-2,7b,12a-triazadibenzo(a,g)cyclonona(cde)trinden-1-one, 2,3,9,10,11,12-hexahydro-9-methoxy-8-methyl-10-(methylamino)-, (8alpha,9beta,10beta,12alpha)-(+)-
1. Staurosporin
2. 62996-74-1
3. (+)-staurosporine
4. Antibiotic 230
5. Am-2282
6. Antibiotic Am 2282
7. Ccris 3272
8. Antibiotic Am-2282
9. Alkaloid Am-2282 From Streptomyces
10. H88epa0a3n
11. Chembl388978
12. Chebi:15738
13. C28h26n4o3
14. 8,12-epoxy-1h,8h-2,7b,12a-triazadibenzo(a,g)cyclonona(cde)trinden-1-one, 2,3,9,10,11,12-hexahydro-9-methoxy-8-methyl-10-(methylamino)-, (8alpha,9beta,10beta,12alpha)-(+)-
15. (5s,6r,7r,9r)-6-methoxy-5-methyl-7-(methylamino)-6,7,8,9,15,16-hexahydro-5h,14h-17-oxa-4b,9a,15-triaza-5,9-methanodibenzo[b,h]cyclonona[jkl]cyclopenta[e]-as-indacen-14-one
16. (5s,6r,7r,9r)-6-methoxy-5-methyl-7-methylamino-6,7,8,9,15,16-hexahydro-5h,14h-5,9-epoxy-4b,9a,15-triazadibenzo[b,h]cyclonona[1,2,3,4-jkl]cyclopenta[e]-as-indacen-14-one
17. Unii-h88epa0a3n
18. Gnf-pf-1389
19. Methoxy-methyl-(methylamino)[?]one
20. Sr-00000001485
21. Cgp-39360
22. Dtxsid6041131
23. Staurosporin, 4
24. 1nvr
25. 1stc
26. 1xbc
27. 1xjd
28. 1yhs
29. 2gcd
30. Staurosporine, 8
31. Cgp 39360
32. Staurosporine & Tnf
33. 1q3d
34. 1sm2
35. 2dq7
36. Staurosporine [mi]
37. Molmap_000047
38. Schembl8157
39. Cbiol_001978
40. Bspbio_001146
41. Gtpl346
42. Bdbm2579
43. Megxm0_000307
44. 1u59
45. Bcpp000063
46. Bio1_000264
47. Bio1_000753
48. Bio1_001242
49. Hms1990j07
50. Hms3650b17
51. Ex-a1777
52. Zinc3814434
53. Am2282
54. Mfcd18252446
55. Nsc755774
56. S1421
57. Staurosporin And Antibiotic Am-2282
58. Akos015897119
59. Am 2282
60. Ccg-208052
61. Db02010
62. Nsc-755774
63. Qtl1_000078
64. Ncgc00162400-01
65. Ncgc00162400-02
66. Ncgc00162400-03
67. Ncgc00162400-04
68. Ncgc00162400-05
69. Ncgc00162400-06
70. Ncgc00162400-09
71. 9,13-epoxy-1h,9h-diindolo(1,2,3-gh:3',2',1'-lm)pyrrolo(3,4-j)(1,7)-benzodiazonin-1-one, 2,3,10,11,12,13-hexahydro-10-methoxy-9-methyl-11-(methylamino)-, (9s-(9alpha,10beta,11beta,13alpha)-
72. Ac-35765
73. Hy-15141
74. Staurosporine & Tumor Necrosis Factor (tnf)
75. Staurosporine 100 Microg/ml In Acetonitrile
76. T4000
77. Q5957181
78. Sr-00000001485-4
79. Brd-k17953061-001-02-8
80. Brd-k17953061-001-04-4
81. Brd-k17953061-001-05-1
82. Brd-k17953061-001-08-5
83. Brd-k17953061-001-10-1
84. Brd-k17953061-001-11-9
85. (2s,3r,4r,6r)-3-methoxy-2-methyl-4-(methylamino)-29-oxa-1,7,17-triazaoctacyclo[12.12.2.1^{2,6}.0^{7,28}.0^{8,13}.0^{15,19}.0^{20,27}.0^{21,26}]nonacosa-8(13),9,11,14(28),15(19),20(27),21(26),22,24-nonaen-16-one
86. (2s,3r,4r,6r)-3-methoxy-2-methyl-4-(methylamino)-29-oxa-1,7,17-triazaoctacyclo[12.12.2.1^{2,6}.0^{7,28}.0^{8,13}.0^{15,19}.0^{20,27}.0^{21,26}]nonacosa-8,10,12,14(28),15(19),20(27),21,23,25-nonaen-16-one
87. (2s,3r,4r,6r)-3-methoxy-2-methyl-4-(methylamino)-29-oxa-1,7,17-triazaoctacyclo[12.12.2.12,6.07,28.08,13.015,19.020,27.021,26]nonacosa-8,10,12,14,19,21,23,25,27-nonaen-16-one
88. (5s,6r,7r,9r)-6-methoxy-5-methyl-7-(methylamino)-6,7,8,9,15,16-hexahydro-5h,14h-5,9-epoxy-4b,9a,15-triazadibenzo[b,h]cyclonona[1,2,3,4-jkl]cyclopenta[e]-as-indacen-14-one
89. (9s,10r,11r,13r)- 2,3,10,11,12,13-hexahydro-10-methoxy-9-methyl-11-(methylamino)-9,13-epoxy-1h,9h-diindolo(1,2,3-gh:3',2',1'-lm)pyrrolo(3,4-j)(1,7)benzodiazonin-1-one
90. [9s-(9?,10?,11?,13?)]-2,3,10,11,12,13-hexahydro-10-methoxy-9-methyl-11-(methylamino)-9,13-epoxy-1h,9h-diindolo[1,2,3-gh:3',2',1'-lm]pyrrolo[3,4-j][1,7]benzodiazonin-1-one
91. 109189-95-9
92. 9,13-epoxy-1h,9h-diindolo[1,2,3-gh:3',2',1'-lm]pyrrolo[3,4-j][1,7]benzodiazonin-1-one, 2,3,10,11,12,13-hexahydro-10-methoxy-9-methyl-11-(methylamino)-, (9s,10r,11r,13r)- & Tumor Necrosis Factor (tnf)
93. 9,13-epoxy-1h,9h-diindolo[1,2,3-gh:3',2',1'-lm]pyrrolo[3,4-j][1,7]benzodiazonin-1-one, 2,3,10,11,12,13-hexahydro-10-methoxy-9-methyl-11-(methylamino)-, (9s,10r,11r,13r)- (9ci)
94. 9,13-epoxy-1h,9h-diindolo[1,2,3-gh:3',2',1'-lm]pyrrolo[3,4-j][1,7]benzodiazonin-1-one, 2,3,10,11,12,13-hexahydro-10-methoxy-9-methyl-11-(methylamino)-, [9s-(9alpha,10beta,11beta,13alpha)]-
| Molecular Weight | 466.5 g/mol |
|---|---|
| Molecular Formula | C28H26N4O3 |
| XLogP3 | 3.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Exact Mass | 466.20049070 g/mol |
| Monoisotopic Mass | 466.20049070 g/mol |
| Topological Polar Surface Area | 69.4 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 0 |
| Complexity | 901 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
