


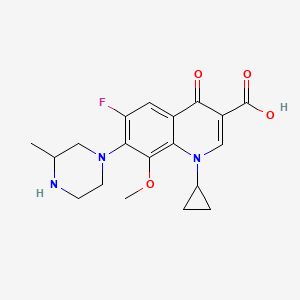

1. 1-cyclopropyl-1,4-dihydro-6-fluoro-8-methoxy-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylic Acid
2. Am 1155
3. Am-1155
4. Bms 206584
5. Bms-206584
6. Bms206584
7. Cg 5501
8. Gatifloxacine
9. Tequin
10. Zymar
1. 112811-59-3
2. Tequin
3. Gatiflo
4. Zymar
5. Am-1155
6. Zymaxid
7. Gatifloxacin Anhydrous
8. Am 1155
9. Zymer
10. Cg 5501
11. 1-cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylic Acid
12. 1-cyclopropyl-6-fluoro-8-methoxy-7-(3-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid
13. Bms-206584
14. Gtfx
15. Bms 206584-01
16. Pd 135432
17. 1-cyclopropyl-1,4-dihydro-6-fluoro-8-methoxy-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylic Acid
18. Gatifloxacin Hydrate
19. Gatifloxacin (inn)
20. Chembl31
21. 3-quinolinecarboxylic Acid, 1-cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-(3-methyl-1-piperazinyl)-4-oxo-
22. Nsc-758701
23. 1-cyclopropyl-6-fluoro-8-methoxy-7-(3-methylpiperazin-1-yl)-4-oxoquinoline-3-carboxylic Acid
24. Pd-135432
25. Chebi:5280
26. Gatilox
27. Gatiquin
28. Gatispan
29. 160738-57-8
30. Gaity
31. Cg5501
32. Mfcd00895399
33. 81485y3a9a
34. Gatifloxin
35. Ncgc00068236-02
36. Gatifloxacin 100 Microg/ml In Acetonitrile
37. Gatifloxacin [inn]
38. 1-cyclopropyl-6-fluoro-8-methoxy-7-(3-methylpiperazin-1-yl)-4-oxo-quinoline-3-carboxylic Acid
39. Dsstox_cid_25704
40. Dsstox_rid_81076
41. Dsstox_gsid_45704
42. Gatifloxacin [usan:inn]
43. Bms-206584-01
44. Bonoq
45. Gatifloxacin (sesquihydrate)
46. Tymer
47. 1-cyclopropyl-6-fluoro- 8-methoxy-7-(3-methylpiperazin-1-yl)- 4-oxo-quinoline-3-carboxylic Acid
48. 3-quinolinecarboxylic Acid,1-cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-(3-methyl-1-piperazinyl)-4-oxo-
49. Smr000043336
50. Gatifloxacin (tn)
51. Cas-112811-59-3
52. Zymer (tn)
53. 1-cyclopropyl-6-fluoro-8-methoxy-7-
54. Sr-01000610458
55. (3-methylpiperazin-1-yl)-4-oxo-1,4-
56. Bms-20658401
57. Gatifloxacino
58. Gatifloxacinum
59. Gatifloxcin
60. Tequin In Dextrose 5% In Plastic Container
61. Am-1155 (*sesquihydrate*)
62. Gatifloxacin & Gamma Interferon
63. Unii-81485y3a9a
64. Gatifloxacin,(s)
65. (+-)-1-cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylic Acid
66. Cg-5501
67. Kinome_3137
68. Spectrum_001909
69. Cpd000043336
70. Pd135432
71. Spectrum2_000487
72. Spectrum3_000999
73. Spectrum4_001127
74. Spectrum5_001468
75. Gatifloxacin [mi]
76. Schembl22591
77. Bspbio_002697
78. Kbiogr_001613
79. Kbioss_002448
80. 1-cyclopropyl-6-fluoro-8-methoxy-7-(3-methylpiperazin-1yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid
81. Mls000040259
82. Mls000759493
83. Mls006011836
84. Gatifloxacin [who-dd]
85. Spectrum1504272
86. Spbio_000353
87. Gatifloxacin Sesquihydrate,(s)
88. Dtxsid5045704
89. Gtpl10816
90. Kbio2_002442
91. Kbio2_005010
92. Kbio2_007578
93. Kbio3_001917
94. Hms1922j15
95. Hms2090k10
96. Hms2093g06
97. Hms2233d20
98. Hms3259p06
99. Hms3372j10
100. Hms3372j12
101. Hms3715n03
102. Pharmakon1600-01504272
103. Albb-028535
104. Amy17781
105. Bcp13408
106. Rkl10068
107. Tox21_110984
108. Bbl010485
109. Bdbm50117914
110. Ccg-39529
111. Nsc758701
112. S1340
113. Stk801620
114. C19h22fn3o4.1.5h2o
115. Akos004119932
116. Akos016340697
117. Tox21_110984_1
118. Ac-1944
119. Cs-1841
120. Db01044
121. Ks-1066
122. Nc00702
123. Nsc 758701
124. Gatifloxacin 100 Microg/ml In Methanol
125. Ncgc00068236-03
126. Ncgc00068236-04
127. Ncgc00068236-05
128. Ncgc00068236-06
129. Ncgc00068236-07
130. Ncgc00068236-08
131. Ncgc00095126-01
132. Ncgc00095126-02
133. Ncgc00178525-01
134. Hy-10581
135. Sbi-0206764.p001
136. Db-019145
137. Ft-0626635
138. Ft-0631189
139. Ft-0668952
140. G0325
141. C07661
142. D08011
143. G-2380
144. Ab00171654-13
145. Ab00171654-14
146. Ab00171654_16
147. Ab00171654_17
148. 811g593
149. A802657
150. Gatifloxacin, Antibiotic For Culture Media Use Only
151. Q2365016
152. Sr-01000610458-2
153. Sr-01000610458-3
154. Brd-a74980173-001-02-8
155. Brd-a74980173-001-06-9
156. (+/-)-1-cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylic Acid
157. 1-cyclopropyl-6-6-fluoro-1,4-dihydro-8-methoxy-7-(3-methylpiperazin-1-yl)-4-oxo-3-quinolinecarboxylic Acid
158. 1-cyclopropyl-6-6-fluoro-1,4-dihydro-8-methoxy-7-(3methylpiperazin-1-yl)-4-oxo-3-quinolinecarboxylic Acid
159. 1-cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-(3-methylpiperazin-1-yl)-4-oxo-3-quinolinecarboxylic Acid
160. 1-cyclopropyl-6-fluoro-8-methoxy-7-(3-methyl-1-piperazinyl)-1,4-dihydro-4-oxoquinoline-3-carboxylic Acid
161. 1-cyclopropyl-6-fluoro-8-methoxy-7-(3-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylicacid
162. 1-cyclopropyl-6-fluoro-8-methoxy-7-(3-methylpiperazin-1yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic A
163. 1-cyclopropyl-6-fluoro-8-methoxy-7-(3-methylpiperazino)-4-oxo-1,4-dihydro-3-quinolinecarboxylic Acid
164. 1-cyclopropyl-7-(3-methyl-1-piperazinyl)-6-fluoro-8-methoxy-1,4-dihydro-4-oxo-3-quinolinecarboxylic Acid
| Molecular Weight | 375.4 g/mol |
|---|---|
| Molecular Formula | C19H22FN3O4 |
| XLogP3 | -0.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 4 |
| Exact Mass | 375.15943435 g/mol |
| Monoisotopic Mass | 375.15943435 g/mol |
| Topological Polar Surface Area | 82.1 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 653 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Gatifloxacin |
| PubMed Health | Gatifloxacin (Into the eye) |
| Drug Classes | Antibiotic |
| Drug Label | ZYMAXID sterile ophthalmic solution is an 8-methoxyfluoroquinolone anti-infective for the treatment of bacterial conjunctivitis. Its chemical name is ()-1-Cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolinecarb... |
| Active Ingredient | Gatifloxacin |
| Dosage Form | Solution/drops; Solution |
| Route | Ophthalmic; ophthalmic |
| Strength | 0.5%; 0.3% |
| Market Status | Tentative Approval; Prescription |
| Company | Hi-tech Pharma; Lupin; Hi-tech Pharmacal |
| 2 of 6 | |
|---|---|
| Drug Name | Zymar |
| PubMed Health | Gatifloxacin (Into the eye) |
| Drug Classes | Antibiotic |
| Drug Label | ZYMAXID sterile ophthalmic solution is an 8-methoxyfluoroquinolone anti-infective for the treatment of bacterial conjunctivitis. Its chemical name is ()-1-Cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolinecarb... |
| Active Ingredient | Gatifloxacin |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 0.3% |
| Market Status | Prescription |
| Company | Allergan |
| 3 of 6 | |
|---|---|
| Drug Name | Zymaxid |
| Active Ingredient | Gatifloxacin |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 0.5% |
| Market Status | Prescription |
| Company | Allergan |
| 4 of 6 | |
|---|---|
| Drug Name | Gatifloxacin |
| PubMed Health | Gatifloxacin (Into the eye) |
| Drug Classes | Antibiotic |
| Drug Label | ZYMAXID sterile ophthalmic solution is an 8-methoxyfluoroquinolone anti-infective for the treatment of bacterial conjunctivitis. Its chemical name is ()-1-Cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolinecarb... |
| Active Ingredient | Gatifloxacin |
| Dosage Form | Solution/drops; Solution |
| Route | Ophthalmic; ophthalmic |
| Strength | 0.5%; 0.3% |
| Market Status | Tentative Approval; Prescription |
| Company | Hi-tech Pharma; Lupin; Hi-tech Pharmacal |
| 5 of 6 | |
|---|---|
| Drug Name | Zymar |
| PubMed Health | Gatifloxacin (Into the eye) |
| Drug Classes | Antibiotic |
| Drug Label | ZYMAXID sterile ophthalmic solution is an 8-methoxyfluoroquinolone anti-infective for the treatment of bacterial conjunctivitis. Its chemical name is ()-1-Cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolinecarb... |
| Active Ingredient | Gatifloxacin |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 0.3% |
| Market Status | Prescription |
| Company | Allergan |
| 6 of 6 | |
|---|---|
| Drug Name | Zymaxid |
| Active Ingredient | Gatifloxacin |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 0.5% |
| Market Status | Prescription |
| Company | Allergan |
For the treatment of bronchitis, sinusitis, community-acquired pneumonia, and skin infections (abscesses, wounds) caused by S. pneumoniae, H. influenzae, S. aureus, M. pneumoniae, C. pneumoniae, L. pneumophila, S. pyogenes
FDA Label
Gatifloxacin is a synthetic broad-spectrum 8-methoxyfluoroquinolone antibacterial agent for oral or intravenous administration. is bactericidal and its mode of action depends on blocking of bacterial DNA replication by binding itself to an enzyme called DNA gyrase, which allows the untwisting required to replicate one DNA double helix into two. Notably the drug has 100 times higher affinity for bacterial DNA gyrase than for mammalian. Gatifloxacin is a broad-spectrum antibiotic that is active against both Gram-positive and Gram-negative bacteria. It should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
Topoisomerase II Inhibitors
Compounds that inhibit the activity of DNA TOPOISOMERASE II. Included in this category are a variety of ANTINEOPLASTIC AGENTS which target the eukaryotic form of topoisomerase II and ANTIBACTERIAL AGENTS which target the prokaryotic form of topoisomerase II. (See all compounds classified as Topoisomerase II Inhibitors.)
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Ophthalmic Solutions
Sterile solutions that are intended for instillation into the eye. It does not include solutions for cleaning eyeglasses or CONTACT LENS SOLUTIONS. (See all compounds classified as Ophthalmic Solutions.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01M - Quinolone antibacterials
J01MA - Fluoroquinolones
J01MA16 - Gatifloxacin
S - Sensory organs
S01 - Ophthalmologicals
S01A - Antiinfectives
S01AE - Fluoroquinolones
S01AE06 - Gatifloxacin
Absorption
Well absorbed from the gastrointestinal tract after oral administration with absolute bioavailability of gatifloxacin is 96%
Gatifloxacin undergoes limited biotransformation in humans with less than 1% of the dose excreted in the urine as ethylenediamine and methylethylenediamine metabolites
7-14 hours
The bactericidal action of Gatifloxacin results from inhibition of the enzymes topoisomerase II (DNA gyrase) and topoisomerase IV, which are required for bacterial DNA replication, transcription, repair, and recombination.