



1. Alcohol, Polyvinyl
2. Liquifilm Tears
3. Polyviol
4. Tears, Liquifilm
1. Ethenol
2. Vinyl Alcohol
3. 9002-89-5
4. Hydroxyethene
5. Hydroxyethylene
6. Gohsenol
7. Polyviol
8. Elvanol
9. Mowiol
10. 557-75-5
11. Ethenol, Homopolymer
12. Poly(vinyl Alcohol)
13. Poval
14. Alcotex 17f-h
15. Alkotex
16. Gelvatol
17. Rhodoviol
18. Einecs 209-183-3
19. Vinylalcohol
20. E1203
21. Polydesis
22. Polyvinol
23. Vinalak
24. Vinarol
25. Vinarole
26. Alvyl
27. Covol
28. Lemol
29. Mfcd00081922
30. Gohsenol Gh
31. Lamephil Oj
32. Sloviol R
33. Kuralon Vp
34. Vinacol Mh
35. Vinarol Dt
36. Vinarol St
37. Aracet Apv
38. Enbra Ov
39. Gtohsenol Gl 05
40. Polysizer 173
41. Cipoviol W 72
42. Gohsenol Ah 22
43. Gohsenol Gh 17
44. Gohsenol Gh 20
45. Gohsenol Gh 23
46. Gohsenol Gl 03
47. Gohsenol Gl 05
48. Gohsenol Gl 08
49. Gohsenol Gm 14
50. Gohsenol Gm 14l
51. Gohsenol Gm 94
52. Gohsenol Kh 17
53. Gohsenol Mg 14
54. Gohsenol Nh 05
55. Gohsenol Nh 17
56. Gohsenol Nh 18
57. Gohsenol Nh 20
58. Gohsenol Nh 26
59. Gohsenol Nl 05
60. Gohsenol Nm 14
61. Vinavilol 2-98
62. Elvanol T 25
63. Gosenol Kh-17
64. Sumitex H 10
65. Gelvatol 1-30
66. Gelvatol 1-60
67. Gelvatol 1-90
68. Gelvatol 2060
69. Gelvatol 2090
70. Gelvatol 3-91
71. Gohsenol N 300
72. Gohsenol Nm 114
73. Kurare Poval 120
74. Kurare 217
75. Rhodoviol 4/125
76. Elvanol 5105
77. Gelvatol 20-30
78. Kurare Pva 205
79. Rhodoviol 4-125p
80. Kurare Poval 1700
81. Lemol Gf-60
82. Poval 205s
83. Poval 217s
84. Poval C 17
85. Elvanol 51-05g
86. Elvanol 52-22g
87. Elvanol 73125g
88. Alcotex 88/05
89. Alcotex 88/10
90. Alcotex 99/10
91. Covol 971
92. Elvanol 50-42
93. Elvanol 52-22
94. Elvanol 70-05
95. Elvanol 71-30
96. Elvanol 90-50
97. Mowiol 4-88
98. Poval 117
99. Poval 120
100. Poval 203
101. Poval 205
102. Poval 217
103. Poval 420
104. Rhodoviol 16/200
105. Vinyl Alcohol, Polymers
106. Elvanol 522-22
107. Mowiol 26-88
108. Polyviol W 28/20
109. Rhodoviol R 16/20
110. Lemol 5-88
111. Lemol 5-98
112. Poval 1700
113. Polyviol Mo 5/140
114. Mowiol N 30-88
115. Mowiol N 50-98
116. Mowiol N 50/88
117. Mowiol N 70-98
118. Lemol 12-88
119. Lemol 16-98
120. Lemol 24-98
121. Lemol 30-98
122. Lemol 51-98
123. Lemol 60-98
124. Lemol 75-98
125. Polyviol M 13/140
126. Polyviol W 25/140
127. Polyviol W 40/140
128. Glo 5
129. Pvs 4
130. Polyvinylalcohol
131. Vinol
132. Pva 008
133. Gh 20
134. Gl 02
135. Gl 03
136. Gm 14
137. Nh 18
138. Nm 11
139. Nm 14
140. Pval 45/02
141. Pval 55/12
142. Gohsenol Nl05
143. Warcopolymer A 20
144. Ep 160
145. Vinyl Alcohol Polymer
146. Fh 1500
147. Vinylon Film 2000
148. Vinylon Film 3000
149. Vinol 125
150. Vinol 205
151. Vinol 351
152. Vinol 523
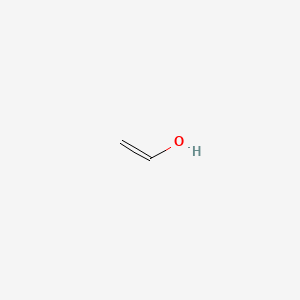
153. Ch2=choh
154. Polyvinylacetate, Hydrolyzed
155. M 13/20
156. Vinylon Film Vf-a 2500
157. Chembl76101
158. Dtxsid8051467
159. Bdbm50473787
160. Nsc108129
161. Vpb 105-2
162. Akos006229012
163. Nsc-108129
164. Ds-002731
165. Ft-0688082
166. Poly(vinyl Alcohol) (fully Hydrolyzed-low M.wt.)
167. Q409591
168. Q27120718
169. Polyvinyl Alcohol, 86-89% Hydrolyzed, Low Molecular Weight
170. Polyvinyl Alcohol, 98-99% Hydrolyzed, Low Molecular Weight
171. Polyvinyl Alcohol, 86-89% Hydrolyzed, Medium Molecular Weight
172. Polyvinyl Alcohol, 87-89% Hydrolyzed, High Molecular Weight
173. Polyvinyl Alcohol, 98-99% Hydrolyzed, High Molecular Weight
174. Polyvinyl Alcohol, 98-99% Hydrolyzed, Medium Molecular Weight
175. Poly(vinyl Alcohol) (fully Hydrolyzed-very Low M.wt.) Mw 7.000-10.000
176. Poly(vinyl Alcohol) N=2,000, (degree Of Saponification Ca. 80mol%)
| Molecular Weight | 44.05 g/mol |
|---|---|
| Molecular Formula | C2H4O |
| XLogP3 | 0.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 44.026214747 g/mol |
| Monoisotopic Mass | 44.026214747 g/mol |
| Topological Polar Surface Area | 20.2 Ų |
| Heavy Atom Count | 3 |
| Formal Charge | 0 |
| Complexity | 10.3 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For use as a lubricant to prevent further irritation or to relieve dryness of the eye(s).
NIH; DailyMed. Current Medication Information for ARTIFICIAL TEARS- polyvinyl alcohol solution/ drops (Revised: June 2015). Available from, as of November 23, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2ef16864-97cb-4dfe-9461-cba156144c69
Do not use if imprinted seal on the bottle neck is broken or missing. Do not use if solution changes color or becomes cloudy. To avoid contamination, do not touch tip of container to any surface. Replace cap after using.
NIH; DailyMed. Current Medication Information for ARTIFICIAL TEARS- polyvinyl alcohol solution/ drops (Revised: June 2015). Available from, as of November 23, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2ef16864-97cb-4dfe-9461-cba156144c69
Stop use and ask a doctor if condition persists or increases discontinue use and consult a veterinarian.
NIH; DailyMed. Current Medication Information for ARTIFICIAL TEARS- polyvinyl alcohol solution/ drops (Revised: June 2015). Available from, as of November 23, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2ef16864-97cb-4dfe-9461-cba156144c69
For use as a lubricant to prevent further irritation or to relieve dryness of the eye(s).
Temporarily relieves burning and irritation due to dryness of the eye or from exposure to wind or sun. Lubricates the eyes and helps protect against further eye irritation/dryness.
Absorption
Polyvinyl alcohol is poorly absorbed from gastrointestinal tract, and readily eliminated from the body.
Volume of Distribution
This drug does not accumulate in the body when administered orally.
The fate of poly(vinyl alcohol) (PVA, 195,000 g/mol) was studied in rabbits and nude mice after intraperitoneal (i.p.) administration. In-vivo fluorescence imaging using nude mice allowed for studies of tetramethylrhodamine labeled PVA distribution in the body and tracking the urinary excretion. The excreted PVA was studied in detail after collecting the urine of rabbits over a time period of 28 days. The PVA was separated from the urine by dialysis and analyzed by FTIR spectroscopy, (1)H-NMR spectroscopy, and size exclusion chromatography (SEC). Even after extensive dialysis, it was found that the excreted PVA showed a characteristic brownish color. The spectroscopic techniques revealed that this color was caused by the urine pigment (a metabolite of bilirubin) that could not be separated completely from the PVA. SEC showed unambiguously that the PVA with the very high molar mass had a glomerular permeability in the kidneys. Simultaneously, histological studies of the kidneys and the liver demonstrated that the tissues did not show any obvious damage.
PMID:20119945 Jiang Y et al; J Biomed Mater Res B Appl Biomater 93 (1): 275-84 (2010)
Polyvinyl alcohol (PVA) is a polymer with a wide range of molecular weights and uses. Recently, low molecular weight formulations of PVA have been used as components of contraceptive products designed for intravaginal administration in human females. Previous studies in animals have determined that little or no absorption of PVA occurs from the gastrointestinal (GI) tract. However, there is some concern that PVA of lower molecular weights might be absorbed across membranes of the reproductive tract. Consequently, this work has investigated the absorption of low molecular weight PVA across biological membranes of the reproductive and GI tracts of Fischer 344 rats. Oral administration of ten consecutive daily doses of (14)C PVA resulted in little apparent absorption of the dose from the GI tract. In contrast, intravaginal administration of (14)C PVA resulted in increasing concentrations of PVA-derived radioactivity in major tissues following one, three or ten daily doses of the estimated human dose of 3 mg/kg. PVA-derived radioactivity was concentrated mainly in the liver, reaching a peak greater than 1750 ng equivalents/g tissue 24 hours following ten daily doses. Over 300 ng equivalents/g tissue were still present in the liver 30 days following the last dose.
PMID:2340195 Sanders JM, Matthews HB; Hum Exp Toxicol 9 (2): 71-7 (1990)
/Researchers/ report on the distribution of PVA of low (mol. wt 37,000), medium (mol. wt 133,000) and high (mol. wt 195,000) grades following 4 weeks of daily subcutaneous injection of 1 mL of 5% solutions to female Holtzman rats. Polymers of medium and high molecular weights were found in the tissues of the adrenal medulla, spleen, myocardium, liver and kidney. Low molecular weight polymers were not found in any tissues.
DeMerlis CC, Schoneker DR; Food Chem Toxicol 41 (3): 319-26 (2003)
PVA sponges (Ivalon) implanted into guinea pigs or rats appeared to disintegrate or deform, suggestive of phagocytosis by macrophages and giant cells or resorption, but there was no indication of the ultimate fate of the sponges or the cellular PVA. Therefore it would seem that while subcutaneously injected PVA may undergo bioaccumulation and implanted PVA undergo partial resorption, intravenous or orally administered PVA is quickly eliminated.
DeMerlis CC, Schoneker DR; Food Chem Toxicol 41 (3): 319-26 (2003)
For more Absorption, Distribution and Excretion (Complete) data for POLYVINYL ALCOHOL (7 total), please visit the HSDB record page.
When injected intravenously, polyvinyl alcohol has a half-life of 90 min. Intraocularly, in eye drop form, the half-life is 7.2 minutes.
As a synthetic resin with hydrophilic properties, it increases the persistence of tear film and therefore lubricates and soothes dry/irritated eyes.