






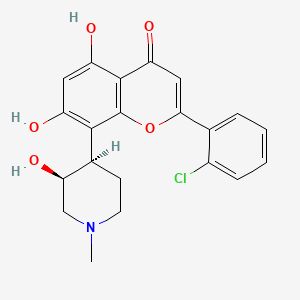

1. (-)cis-5,7-dihydroxy-2-(2-chlorophenyl)-8-(4-(3-hydroxy-1-methyl)piperidinyl)-4h-1-benzopyran-4-one
2. Flavopiridol
3. Hmr 1275
4. L 868275
5. L-868275
6. L86-8275
1. Flavopiridol
2. 146426-40-6
3. Alvocidib Freebase
4. L86-8275
5. L 868275
6. L-868275
7. Flavopiridol (alvocidib)
8. Hmr-1275
9. Hmr 1274
10. 131740-09-5
11. L 86-8275
12. 2-(2-chlorophenyl)-5,7-dihydroxy-8-[(3s,4r)-3-hydroxy-1-methylpiperidin-4-yl]-4h-chromen-4-one
13. 2-(2-chlorophenyl)-5,7-dihydroxy-8-[(3s,4r)-3-hydroxy-1-methylpiperidin-4-yl]chromen-4-one
14. Chembl428690
15. 45ad6x575g
16. 2-(2-chlorophenyl)-5,7-dihydroxy-8-((3s,4r)-3-hydroxy-1-methylpiperidin-4-yl)-4h-chromen-4-one
17. 2-(2-chloro-phenyl)-5,7-dihydroxy-8-(3-hydroxy-1-methyl-piperidin-4-yl)-4h-benzopyran-4-one
18. Nsc 649890 Hcl
19. Mdl-107826a
20. 2-(2-chlorophenyl)-5,7-dihydroxy-8-[(3s,4r)-3-hydroxy-1-methyl-4-piperidyl]chromen-4-one
21. Alvocidib [inn]
22. Flavo
23. Flavoperidol
24. 4h-1-benzopyran-4-one, 2-(2-chlorophenyl)-5,7-dihydroxy-8-[(3s,4r)-3-hydroxy-1-methyl-4-piperidinyl]-
25. Alvocidib [usan]
26. L868275
27. Alvocidib [usan:inn]
28. Alvocidibum
29. Unii-45ad6x575g
30. Ccris 9399
31. 4h-1-benzopyran-4-one, 2-(2-chlorophenyl)-5,7-dihydroxy-8-((3s,4r)-3-hydroxy-1-methyl-4-piperidinyl)-
32. (-)cis-5,7-dihydroxy-2-(2-chlorophenyl)-8-(4-(3-hydroxy-1-methyl)piperidinyl)-4h-1-benzopyran-4-one
33. Nsc649890
34. 1c8k
35. 1e1y
36. Alvocidib (usan/inn)
37. Flavopiridol [mi]
38. Alvocidib [mart.]
39. Alvocidib [who-dd]
40. Schembl3652
41. Flavopiridol Hcl; Alvocidib
42. Bdbm5655
43. Gtpl5680
44. Chebi:47344
45. Dtxsid20904970
46. Ex-a1901
47. Flavopiridol,alvocidib, Hmr-1275
48. Hmr-1274
49. Mfcd20501884
50. Nsc799330
51. S1230
52. Zinc21288966
53. Am84422
54. Ccg-268666
55. Db03496
56. Nsc-799330
57. (-)-cis-5,7-dihydroxy-2-(2-chlorophenyl)-8-(4-(3-hydroxy-1-methyl)piperidinyl)-4h-1-benzopyran-4-one
58. 4h-1-benzopyran-4-one, 2-(2-chlorophenyl)-5,7-dihydroxy-8-((3r,4s)-3-hydroxy-1-methyl-4-piperidinyl)-, Rel-(-)-
59. 4h-1-benzopyran-4-one, 2-(2-chlorophenyl)-5,7-dihydroxy-8-(3-hydroxy-1-methyl-4-piperidinyl)-, Cis-(-)-
60. As-74761
61. Hy-10005
62. A25160
63. D09868
64. J-008219
65. Q4063441
66. Brd-k87909389-001-01-2
67. (-)-cis-2-(2-chlorophenyl)-5,7-dihydroxy-8-(3-hydroxy-1-methylpiperidin-4-yl)-4h-1-benzopyran-4-one
68. 2-(2-chlorophenyl)-5,7-dihydroxy-8-((3s,4r)-3-hydroxy-1-methylpiperidin-4-yl)-4h-chromen-4-one;alvocidib
69. 4h-1-benzopyran-4-one, 2-(2-chlorophenyl)-2,3-dihydro-5,7-dihydroxy-8-[(3s,4r)-3-hydroxy-1-methyl-4-piperidinyl]-
70. 5,7-dihydroxy-2-(2-chlorophenyl)-8-(1-methyl-3beta-hydroxypiperidin-4beta-yl)-4h-1-benzopyran-4-one
| Molecular Weight | 401.8 g/mol |
|---|---|
| Molecular Formula | C21H20ClNO5 |
| XLogP3 | 3.3 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | 401.1030004 g/mol |
| Monoisotopic Mass | 401.1030004 g/mol |
| Topological Polar Surface Area | 90.2 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 628 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in esophageal cancer, leukemia (lymphoid), lung cancer, liver cancer, and lymphoma (unspecified).
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Protein Kinase Inhibitors
Agents that inhibit PROTEIN KINASES. (See all compounds classified as Protein Kinase Inhibitors.)
Growth Inhibitors
Endogenous or exogenous substances which inhibit the normal growth of human and animal cells or micro-organisms, as distinguished from those affecting plant growth (= PLANT GROWTH REGULATORS). (See all compounds classified as Growth Inhibitors.)
Flavopiridol has known human metabolites that include (2S,3S,4S,5R)-6-[2-(2-chlorophenyl)-5-hydroxy-8-[(3S,4R)-3-hydroxy-1-methylpiperidin-4-yl]-4-oxochromen-7-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Inhibits cyclin-dependent kinases, arresting cell division and causing apoptosis in non-small lung cancer cells.
