






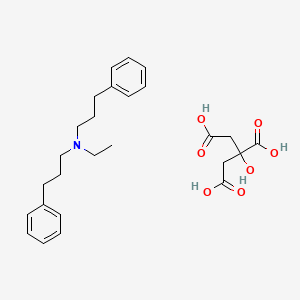

1. Alverine
2. Alverine Hydrochloride
3. Spasmaverine
4. Spasmonal
1. 5560-59-8
2. Alverine (citrate)
3. Antispasmin
4. Spacolin
5. Gamatran Citrate
6. Nsc 35459
7. Spasmaverine
8. Alverine Citrate Salt
9. Calmabel
10. Profenine
11. Prophelan
12. Proverine
13. Profenil Citrate
14. Nci 85x
15. Alverine Citrate [usan]
16. Nsc-35459
17. Phenpropamine Citrate
18. N-ethyl-3,3'-diphenyldipropylamine Citrate (1:1)
19. 9jfb58yk1e
20. Mls000069524
21. 5560-59-8 (citrate)
22. Chebi:53785
23. N-ethyl-3-phenyl-n-(3-phenylpropyl)propan-1-amine 2-hydroxypropane-1,2,3-tricarboxylate
24. Dipropylamine, N-ethyl-3,3'-diphenyl-, Citrate (1:1)
25. Ncgc00017047-01
26. Smr000058631
27. Cas-5560-59-8
28. Alverine Citrate (usan)
29. Benzenepropanamine, N-ethyl-n-(3-phenylpropyl)-, 2-hydroxy-1,2,3-propanetricarboxylate (1:1)
30. Dsstox_cid_25562
31. Dsstox_rid_80959
32. Dsstox_gsid_45562
33. Alverine Dihydrogen Citrate
34. N-ethyl-3-phenyl-n-(3-phenylpropyl)propan-1-amine;2-hydroxypropane-1,2,3-tricarboxylic Acid
35. Sr-01000003029
36. Unii-9jfb58yk1e
37. Alverine Citrate [usan:nf]
38. Prestwick_912
39. Einecs 226-929-3
40. Alverinecitrate
41. Opera_id_1477
42. Dipropylamine, N-ethyl-3,3'-diphenyl-, Citrate
43. N-ethyl-bis(3-phenylpropyl)amin-dihydrogencitrat
44. Mls001148462
45. Mls002207301
46. Mls002222279
47. Mls004712036
48. Alverine Citrate [mi]
49. Schembl352008
50. Spectrum1500109
51. Regid_for_cid_21718
52. Chembl1408594
53. Dtxsid3045562
54. Alverine Citrate [mart.]
55. Alverine Citrate [who-dd]
56. Hms1568d09
57. Hms1920a17
58. Hms2091g17
59. Hms2095d09
60. Hms2232d03
61. Hms3371i14
62. Hms3651f18
63. Hms3712d09
64. Hms3885o05
65. Pharmakon1600-01500109
66. Hy-b0500
67. Tox21_110757
68. Dipropylamine,3'-diphenyl-, Citrate
69. Mfcd00035086
70. Nsc755859
71. S3054
72. Akos015895657
73. Tox21_110757_1
74. Alverine Citrate [ep Monograph]
75. Ccg-212472
76. Nsc-755859
77. Ncgc00017047-02
78. Ncgc00017047-03
79. Ncgc00021122-08
80. Ncgc00094581-01
81. Ncgc00094581-02
82. As-56723
83. Db-052764
84. N-ethyl-3,3'-diphenyldipropylamine Citrate
85. Dipropylamine,3'-diphenyl-, Citrate (1:1)
86. Ft-0622247
87. Sw197030-3
88. A16396
89. D02877
90. D84224
91. A830720
92. Sr-01000003029-2
93. Sr-01000003029-4
94. Q27124210
95. Benzenepropanamine, 2-hydroxy-1,2,3-propanetricarboxylate (1:1)
96. Citric Acid; N-ethyl-3-phenyl-n-(3-phenylpropyl)propan-1-amine
97. 2-hydroxypropane-1,2,3-tricarboxylic Acid; Ethylbis(3-phenylpropyl)amine
98. Alverine Citrate Salt, European Pharmacopoeia (ep) Reference Standard
99. Alverine For Peak Identification, European Pharmacopoeia (ep) Reference Standard
| Molecular Weight | 473.6 g/mol |
|---|---|
| Molecular Formula | C26H35NO7 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 14 |
| Exact Mass | 473.24135246 g/mol |
| Monoisotopic Mass | 473.24135246 g/mol |
| Topological Polar Surface Area | 135 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 442 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Parasympatholytics
Agents that inhibit the actions of the parasympathetic nervous system. The major group of drugs used therapeutically for this purpose is the MUSCARINIC ANTAGONISTS. (See all compounds classified as Parasympatholytics.)
