
1. 4 Aminosalicylic Acid
2. Acid, Aminosalicylic
3. Alumino 4 Aminosalicylic Acid
4. Alumino-4-aminosalicylic Acid
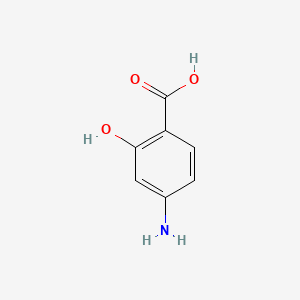
5. Aminosalicylic Acid
6. P Aminosalicylic Acid
7. P Aminosalicylic Acid Monolithium Salt
8. P Aminosalicylic Acid Monopotassium Salt
9. P Aminosalicylic Acid Monosodium Salt
10. P-aminosalicylic Acid
11. P-aminosalicylic Acid Monolithium Salt
12. P-aminosalicylic Acid Monopotassium Salt
13. P-aminosalicylic Acid Monosodium Salt
14. P-aminosalicylic Acid, Aluminum (2:1) Salt
15. P-aminosalicylic Acid, Calcium (2:1) Salt
16. P-aminosalicylic Acid, Monosodium Salt, Dihydrate
17. Pamisyl
18. Para Aminosalicylic Acid
19. Para-aminosalicylic Acid
20. Rezipas
1. 4-amino-2-hydroxybenzoic Acid
2. 65-49-6
3. Aminosalicylic Acid
4. P-aminosalicylic Acid
5. Rezipas
6. Para-aminosalicylic Acid
7. Aminopar
8. Pamisyl
9. Parasal
10. Paser
11. Parasalindon
12. Deapasil
13. Apacil
14. Gabbropas
15. Paramycin
16. Parasalicil
17. Pasnodia
18. Aminox
19. Benzoic Acid, 4-amino-2-hydroxy-
20. Entepas
21. Osacyl
22. Pamacyl
23. Pasalon
24. Pasara
25. Pasdium
26. Pasmed
27. Pasolac
28. Propasa
29. Pasem
30. Pasa
31. 2-hydroxy-4-aminobenzoic Acid
32. Apas
33. Pask
34. Para-pas
35. Sanipirol-4
36. Para-amino Salicylic Acid
37. Hellipidyl
38. Pascorbic
39. Pas-c
40. Pas (acid)
41. 4-aminosalicylate
42. Pas
43. Kyselina P-aminosalicylova
44. Aminosalicylate
45. Salicylic Acid, 4-amino-
46. 3-hydroxy-4-carboxyaniline
47. Amino-pas
48. Sanipriol-4
49. Nih 2939
50. Nsc 2083
51. Mfcd00007789
52. 4-asa
53. Aminosalicylate Sodium
54. Aminosalicylic Acid [usp]
55. A 1909
56. Benzoic Acid, 4-aminohydroxy-
57. 4-amino-2-hydroxy-benzoic Acid
58. P-amino Salicylic Acid
59. 4-amino Salicylic Acid
60. Nsc-2083
61. Mls000069418
62. Chebi:27565
63. Nsc2083
64. 5b2658e0n2
65. Aminosalyl
66. Helipidyl
67. Smr000059110
68. Paser Granules
69. Aminosalicylic Acid (usp)
70. Paskalium
71. Mls000069579
72. Pas (van)
73. 4-aminosalicylicacid
74. Aminosalicylic Acid Resin Complex
75. Hsdb 3203
76. 4-amino-salicylic Acid
77. Kyselina P-aminosalicylova [czech]
78. Sr-01000002990
79. Smr000058830
80. Einecs 200-613-5
81. Brn 0473071
82. P.a.s
83. Aminosalicylic
84. Granupas
85. Ai3-50142
86. Unii-5b2658e0n2
87. Salicylic Acid Usp
88. Isonicotinic Acid Hydrazide P-aminosalicylate Salt
89. Pamisyl (tn)
90. Paser (tn)
91. 4-aminosalicyclic Acid
92. Spectrum_000042
93. .gamma.-aminosalicylate
94. (mixture Of Tautomers)
95. Opera_id_614
96. Spectrum2_000001
97. Spectrum3_000297
98. Spectrum4_000145
99. Spectrum5_000804
100. Para-amino-salicyclic Acid
101. Wln: Zr Cq Dvq
102. Salicyclic Acid, 4-amino-
103. Schembl2262
104. Chembl1169
105. Bspbio_001834
106. Kbiogr_000590
107. Kbioss_000422
108. Zinc922
109. 4-14-00-01967 (beilstein Handbook Reference)
110. Mls001148121
111. 4-aminosalicylic Acid, 99%
112. Bidd:gt0175
113. Divk1c_000350
114. 4-amino-2-hydroxobenzoic Acid
115. Spbio_000001
116. P-aminosalicylic Acid Standard
117. Dtxsid2022591
118. Bdbm48319
119. Hms501b12
120. Kbio1_000350
121. Kbio2_000422
122. Kbio2_002990
123. Kbio2_005558
124. Kbio3_001334
125. 4-azanyl-2-oxidanyl-benzoic Acid
126. Cid_11988145
127. Ninds_000350
128. Aminosalicylic Acid [hsdb]
129. Hms2090i07
130. Hms2093l14
131. Hms2236i04
132. Hms3371a17
133. Hms3715m08
134. Kuc106682n
135. Aminosalicylic Acid [vandf]
136. P-aminosalicylic Acid [mi]
137. Aminosalicylic Acid [mart.]
138. Amy31099
139. Bcp18565
140. Hy-i0447
141. Aminosalicylic Acid [usp-rs]
142. Aminosalicylic Acid [who-dd]
143. Ccg-39969
144. S5211
145. Stl163955
146. Akos000121200
147. Cs-w023102
148. Db00233
149. Pb47849
150. 4-amino,2-hydroxy-benzoic Acid
151. Idi1_000350
152. Aminosalicylic Acid [orange Book]
153. Ncgc00018110-01
154. Ncgc00018110-02
155. Ncgc00018110-03
156. Ncgc00018110-04
157. Ac-12894
158. As-11043
159. Sy001079
160. Aminosalicylic Acid [usp Monograph]
161. Ksc-11-207-13
162. Sbi-0051279.p003
163. Sbi-0051279.p004
164. Db-054818
165. Mesalazine Impurity E [ep Impurity]
166. A0420
167. Ft-0617609
168. Ft-0689453
169. C02518
170. D00162
171. Neopasalate Component Aminosalicylic Acid
172. P17508
173. Ab00051913-20
174. Aminosalicylic Acid Component Of Neopasalate
175. Q229924
176. Q-200437
177. Sr-01000002990-4
178. Sr-01000002990-6
179. 4-aminosalicylic Acid, Vetec(tm) Reagent Grade, 99%
180. Z90121065
181. F2191-0245
182. Para-aminosalicylic Acid;aminosalicylic Acid;4-aminosalicylate
183. Aminosalicylic Acid, United States Pharmacopeia (usp) Reference Standard
184. 4-aminosalicylic Acid, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 153.14 g/mol |
|---|---|
| Molecular Formula | C7H7NO3 |
| XLogP3 | 1.3 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | 153.042593085 g/mol |
| Monoisotopic Mass | 153.042593085 g/mol |
| Topological Polar Surface Area | 83.6 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 160 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Paser |
| PubMed Health | Aminosalicylic Acid (By mouth) |
| Drug Classes | Antitubercular |
| Drug Label | PASER granules are a delayed release granule preparation of aminosalicylic acid (p-aminosalicylic acid; 4-aminosalicylic acid) for use with other anti-tuberculosis drugs for the treatment of all forms of active tuberculosis due to susceptible strains... |
| Active Ingredient | Aminosalicylic acid |
| Dosage Form | Granule, delayed release |
| Route | Oral |
| Strength | 4gm/packet |
| Market Status | Prescription |
| Company | Jacobus |
| 2 of 2 | |
|---|---|
| Drug Name | Paser |
| PubMed Health | Aminosalicylic Acid (By mouth) |
| Drug Classes | Antitubercular |
| Drug Label | PASER granules are a delayed release granule preparation of aminosalicylic acid (p-aminosalicylic acid; 4-aminosalicylic acid) for use with other anti-tuberculosis drugs for the treatment of all forms of active tuberculosis due to susceptible strains... |
| Active Ingredient | Aminosalicylic acid |
| Dosage Form | Granule, delayed release |
| Route | Oral |
| Strength | 4gm/packet |
| Market Status | Prescription |
| Company | Jacobus |
Antitubercular Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
EXPTL USE: LIPID LOWERING AGENT. 6 G GIVEN FOR 4 WK. IT WAS CONCLUDED THAT IT LOWERS ELEVATED SERUM TRIGLYCERIDE LEVELS AS WELL AS ELEVATED SERUM CHOLESTEROL LEVELS.
VESSBY ET AL; PARA-AMINOSALICYLIC ACID AS A LIPID LOWERING AGENT; CLIN PHARMACOL THER 23 (JUN) 651-7 (1978)
USED ALONE, IT CAN SOMETIMES SUCCESSFULLY MANAGE /TUBERCULOSIS/...BUT RESISTANCE EMERGES & ALSO TOXICITY LIMITS THE DOSE. THEREFORE, PAS IS NEARLY ALWAYS USED IN COMBINATION WITH 1 OR 2 OTHER ANTITUBERCULAR DRUGS. ...PAS SUPPORTS THE OTHER DRUGS & DELAYS THE EMERGENCY OF RESISTANCE.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1150
AMINOSALICYLIC ACID...HAS POTENT HYPOLIPIDEMIC ACTION & REDUCES BOTH CHOLESTEROL & TRIGLYCERIDES. HOWEVER IT HAS NOT BEEN WELL TOLERATED BECAUSE OF GI REACTION.
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 161
For more Therapeutic Uses (Complete) data for P-AMINOSALICYLIC ACID (15 total), please visit the HSDB record page.
UNDER NO CIRCUMSTANCES USE SOLN IF ITS COLOR IS DARKER THAN THAT OF FRESHLY PREPD SOLN. ... PREPARE SOLN OF CALCIUM, /POTASSIUM, & SODIUM SALTS/ WITHIN 24 HR OF ADMIN.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1149
FOR THE VARIOUS DEFECTS /FOR EXAMPLE, DEFICIENCY IN ERYTHROCYTE GLUCOSE-6-PHOSPHATE DEHYDROGENASE/ THAT SEEM TO BE SPECIFIC TO PARTICULAR RACES, DIFFERENT DRUGS ELICIT HEMOLYSIS. MOST IMPORTANT OF THESE ARE NITROFURANTOIN, AMINOSALICYLIC ACID...
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 1: A Review of the Literature Published Between 1960 and 1969. London: The Chemical Society, 1970., p. 429
IN PT WITH IMPAIRMENT OF KIDNEY OR OTHER MECHANISMS FOR CONTROLLING PLASMA CONCN, THE DRUG CAN CAUSE HYPERCALCEMIA. IT MAY ALSO CONTRIBUTE TO UROLITHIASIS. /CA SALT/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1149
The most frequent adverse effects of aminosalicylic acid or its salt are GI disturbances including nausea, vomiting, abdominal pain, diarrhea, and anorexia. Rarely, aminosalicylic acid has caused peptic ulcer and gastric hemorrhage. Adverse GI effects may be minimized in some patients by administering the aminosalicylates with meals; however, symptoms may be severe enough to require discontinuation of the drugs. Malabsorption of vitamin B12 folic acid, iron, and lipids has also occurred occasionally in patients receiving aminosalicylic acid or its salt, possibly as the result of increased peristalsis. The manufacturer states that maintenance therapy with vitamin B12 should be considered in patients receiving aminosalicylic acid for longer than 1 month.
McEvoy G.K. (ed.). American Hospital Formulary Service-Drug Information 96. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1996 (Plus Supplements)., p. 400
For more Drug Warnings (Complete) data for P-AMINOSALICYLIC ACID (12 total), please visit the HSDB record page.
For the treatment of tuberculosis
Granupas is indicated for use as part of an appropriate combination regimen for multi-drug resistant tuberculosis in adults and paediatric patients from 28 days of age and older when an effective treatment regimen cannot otherwise be composed for reasons of resistance or tolerability (see section 4. 4).
Consideration should be given to official guidance on the appropriate use of antibacterial agents.
Aminosalicylic acid is an anti-mycobacterial agent used with other anti-tuberculosis drugs (most often isoniazid) for the treatment of all forms of active tuberculosis due to susceptible strains of tubercle bacilli. The two major considerations in the clinical pharmacology of aminosalicylic acid are the prompt production of a toxic inactive metabolite under acid conditions and the short serum half life of one hour for the free drug. Aminosalicylic acid is bacteriostatic against Mycobacterium tuberculosis (prevents the multiplying of bacteria without destroying them). It also inhibits the onset of bacterial resistance to streptomycin and isoniazid.
Antitubercular Agents
Drugs used in the treatment of tuberculosis. They are divided into two main classes: "first-line" agents, those with the greatest efficacy and acceptable degrees of toxicity used successfully in the great majority of cases; and "second-line" drugs used in drug-resistant cases or those in which some other patient-related condition has compromised the effectiveness of primary therapy. (See all compounds classified as Antitubercular Agents.)
J04AA01
J - Antiinfectives for systemic use
J04 - Antimycobacterials
J04A - Drugs for treatment of tuberculosis
J04AA - Aminosalicylic acid and derivatives
J04AA01 - 4-aminosalicylic acid
BIOAVAILABILITY STUDIES ON P-AMINOSALICYLIC ACID & ITS SALTS IN 12 SUBJECTS. COLORIMETRIC ASSAY INDICATED THAT PEAK BLOOD LEVELS OCCURRED @ 0.5, 0.75, 1.5, & 3 HR FOR SODIUM, POTASSIUM, & CALCIUM SALTS & P-AMINOSALICYLIC ACID, RESPECTIVELY.
WAN ET AL; J PHARMACOKINET BIOPHARM 2 (FEB): 1-12 (1974)
URINE EXCRETION DATA SHOWED ABSORPTION TO BE ESSENTIALLY COMPLETE ALTHOUGH RATES OF ABSORPTION DIFFERED.
WAN ET AL; J PHARMACOKINET BIOPHARM 2 (FEB): 1-12 (1974)
Aminosalicyclic acid is readily absorbed from the gastrointestinal tract. A single oral dose of 4 g of the free acid produces maximal concentrations in plasma of about 75 ug/ml within 1.5 to 2 hours. The sodium salt is absorbed even more rapidly. The drug appears to be distributed throughout the total body water and reaches high concentrations in pleural fluid and caseous tissue. However, values in CSF are low, perhaps because of active outward transport.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1164
Over 80% of the drug is excreted in the urine; more than 50% is in the form of the acetylated compound. The largest portion of the remainder is made up of the free acid.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1164
For more Absorption, Distribution and Excretion (Complete) data for P-AMINOSALICYLIC ACID (8 total), please visit the HSDB record page.
Hepatic.
ACETYLATION IS MAJOR ROUTE FOR INACTIVATION OF MANY DRUGS SUCH AS ... P-AMINOSALICYLIC ACID... ENZYMES WHICH CATALYSE THESE REACTIONS, ACETYL COENZYME A:N-ACETYLTRANSFERASES (EC2.3.1.5), ARE LOCATED IN LIVER CYTOSOL.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 545
WHEN ADMIN ORALLY TO MAN IT IS RAPIDLY ABSORBED, & IS EXCRETED IN URINE AS UNCHANGED P-AMINOSALICYLIC ACID & AS ACETYL GLUCURONYL, GLYCYL & GLUTAMINYL CONJUGATES.
Parke, D. V. The Biochemistry of Foreign Compounds. Oxford: Pergamon Press, 1968., p. 176
YIELDS 5-AMINO-2-CARBOXYPHENYL-BETA-D-GLUCURONIDE IN MAN. 4-AMINOCATECHOL IN PSEUDOMONAS. 4-AMINOSALICYLOYLGLUTAMINE & 4-AMINOSALICYLOYLGLYCINE IN MAN. /TABLE/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. A-52
Blood from tuberculosis patients was cultured before, during, and after withdrawal of therapy involving five different drug combinations if isoniazid, thiacetazone, p-aminosalicyclic acid, and streptomycin. The approaches used to detect DNA damage were chromosome aberrations and sister chromatid exchanges (SCEs). A total of 179 subjects were analyzed. In combo these drugs showed synergistic, additive, and antagonistic effects, though they were found to be nonclastogenic individually. Four of the drug combinations, isoniazid plus thiacetazone, isoniazid plus p-aminosalicyclic acid, isoniazid plus thiacetazone plus streptomycin, and isoniazid plus p-aminosalicyclic acid plus streptomycin, induced a significant incr in the frequency of aberrations, whereas isoniazid plus streptomycin did not induce aberrations. In fact, streptomycin appeared to reduce the frequency of aberrations. SCEs were incr in only two patients: one treated with isoniazid plus thiacetazone and the other with isoniazid plus p-aminosalicyclic acid. The frequency of aberrations after withdrawal of therapy was decr; it was slightly higher than the controls, though it was insignificant. The return to normalcy could be due to elimination of damaged cells or the repair of DNA in lymphocytes. Though the drug-induced aberrations do not persist after withdrawal of therapy, the chromosome damaging combo of drugs should be used with caution, because the possibility of meiotic chromosome damage in germ cells (during therapy), which might be passed on to the next generation, cannot be ruled out.
PMID:6205465 Jaju M et al; Teratog Carcinog Mutagen 4 (3): 261-72 (1984)
For more Metabolism/Metabolites (Complete) data for P-AMINOSALICYLIC ACID (9 total), please visit the HSDB record page.
The drug has a half life of about 1 hour, and concentrations in plasma are negligible within 4 to 5 hours after a single conventional dose.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1164
There are two mechanisms responsible for aminosalicylic acid's bacteriostatic action against Mycobacterium tuberculosis. Firstly, aminosalicylic acid inhibits folic acid synthesis (without potentiation with antifolic compounds). The binding of para-aminobenzoic acid to pteridine synthetase acts as the first step in folic acid synthesis. Aminosalicylic acid binds pteridine synthetase with greater affinity than para-aminobenzoic acid, effectively inhibiting the synthesis of folic acid. As bacteria are unable to use external sources of folic acid, cell growth and multiplication slows. Secondly, aminosalicylic acid may inhibit the synthesis of the cell wall component, mycobactin, thus reducing iron uptake by M. tuberculosis.
The antimicrobial activity of aminosalicylic acid is highly specific, and microorganisms other than Mycobacterium tuberculosis are unaffected. Most nontuberculous mycobacteria are not inhibited by the drug.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1164
Aminosalicyclic acid is a structural analog of paraaminobenzoic acid, and its mechanism of action appears to be very similar to that of the sulfonamides. Since the sulfonamides are ineffective against Mycobacterium tuberculosis, and aminosalicyclic is inactive against sulfonamide susceptible bacteria, it is probable that the enzymes responsible for folate biosynthesis in various microorganisms may be quite exacting in their capacity to distinguish various analogs from the true metabolite.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1164