

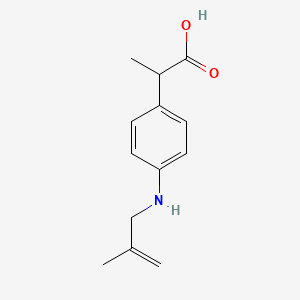

1. 2-(4-((2-methyl-2-propenyl)amino)phenyl)propionic Acid
2. 2-(4-methylallylaminophenyl)propionic Acid
3. Eb 382
4. Eb-382
1. 39718-89-3
2. Alminoprofene
3. Alminoprofeno
4. Alminoprofenum
5. Minalfen
6. Eb-382
7. P-((2-methylallyl)amino)hydratropic Acid
8. Eb 382
9. Brn 2373633
10. 2-(4-((2-methylallyl)amino)phenyl)propansaeure
11. 0255ahr9gj
12. Chebi:31190
13. Benzeneacetic Acid, Alpha-methyl-4-((2-methyl-2-propenyl)amino)-
14. Acide (methallylamino-4 Phenyl)-2 Propionique
15. 2-{4-[(2-methylprop-2-en-1-yl)amino]phenyl}propanoic Acid
16. Alpha-methyl-4-((2-methyl-2-propenyl)amino)benzeneacetic Acid
17. 2-{4-[(2-methylprop-2-en-1-yl)amino]phenyl}propionic Acid
18. Alpha-methyl-4-[(2-methyl-2-propenyl)amino]benzeneacetic Acid
19. 2-(4-((2-methylprop-2-en-1-yl)amino)phenyl)propanoic Acid
20. 2-(4-((2-methylprop-2-en-1-yl)amino)phenyl)propionic Acid
21. 2-(4-methylallylaminophenyl)propionic Acid
22. Refchem:554938
23. 2-(4-((2-methyl-2-propenyl)amino)phenyl)propionic Acid
24. M01ae16
25. (2rs)-2-((4-(2-methylprop-2-en-1-yl)amino)phenyl)propanoic Acid
26. 254-604-6
27. Minalfene
28. 2-(4-((2-methylallyl)amino)phenyl)propanoic Acid
29. Rac Alminoprofen
30. Racalminoprofen-d3
31. 54362-71-9
32. Alminoprofen, (+)-
33. 2-[4-(2-methylprop-2-enylamino)phenyl]propanoic Acid
34. 2-[4-(2-methylallylamino)phenyl]propanoic Acid
35. Alminoprofeno [spanish]
36. Alminoprofen [inn:jan]
37. Alminoprofene [inn-french]
38. Alminoprofenum [inn-latin]
39. Alminoprofeno [inn-spanish]
40. (+/-)-alminoprofen;eb 382; Minalfene
41. (+/-)-alminoprofen
42. Einecs 254-604-6
43. Unii-0255ahr9gj
44. 4-((2-methylallyl)amino)hydratropasaeure
45. 2-(4-(methallylamino)phenyl)propionic Acid
46. Minalfen (tn)
47. Mfcd00866084
48. Acide (methallylamino-4 Phenyl)-2 Propionique [french]
49. Alminoprofen (standard)
50. Alminoprofen [mi]
51. Alminoprofen [inn]
52. Alminoprofen [jan]
53. Alminoprofen (jp17/inn)
54. Propionic Acid, 2-(4-(methallylamino)phenyl)-
55. Schembl26940
56. Alminoprofen [mart.]
57. Alminoprofen [who-dd]
58. Orb1703760
59. Chembl1765293
60. Dtxsid90865968
61. Fphlbgojwpevme-uhfffaoysa-n
62. Bcp11016
63. Uwc97721
64. Hy-17485r
65. ()-alminoprofen;eb 382; Minalfene
66. Akos016014146
67. Db13314
68. Ncgc00522017-01
69. As-78318
70. Da-60933
71. Hy-17485
72. ( Inverted Exclamation Marka)-alminoprofen
73. Cs-0009220
74. Ns00015369
75. D01513
76. F600577
77. Q3612806
| Molecular Weight | 219.28 g/mol |
|---|---|
| Molecular Formula | C13H17NO2 |
| XLogP3 | 3.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 5 |
| Exact Mass | Da |
| Monoisotopic Mass | Da |
| Topological Polar Surface Area | 49.3 |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 255 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects.
M - Musculo-skeletal system
M01 - Antiinflammatory and antirheumatic products
M01A - Antiinflammatory and antirheumatic products, non-steroids
M01AE - Propionic acid derivatives
M01AE16 - Alminoprofen
ATCvet Code
QM - Musculo-skeletal system
QM01 - Antiinflammatory and antirheumatic products
QM01A - Antiinflammatory and antirheumatic products, non-steroids
QM01AE - Propionic acid derivatives
QM01AE16 - Alminoprofen

