

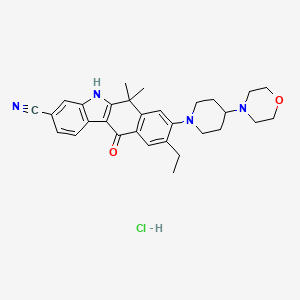

1. Alecensa
2. Alectinib
3. Ch5424802
4. Ro5424802
1. 1256589-74-8
2. Af-802 Hydrochloride
3. Alectinib Hcl
4. Alecensa
5. P9yy73lo6j
6. Alectinib Hydrochloride (jan)
7. Alectinib (hydrochloride)
8. Ch5424802 (hydrochloride)
9. Schembl14991271
10. 5h-benzo[b]carbazole-3-carbonitrile, 9-ethyl-6,11-dihydro-6,6-dimethyl-8-[4-(4-morpholinyl)-1-piperidinyl]-11-oxo-, Hydrochloride (1:1)
11. Alectinib Hydrochloride [jan]
12. 9-ethyl-6,6-dimethyl-8-[4-(morpholin-4-yl)piperidin-1-yl]-11-oxo-6,11-dihydro-5h-benzo[b]carbazole-3-carbonitrile Hydrochloride
13. Unii-p9yy73lo6j
14. Alecensa (tn)
15. 5h-benzo(b)carbazole-3-carbonitrile, 9-ethyl-6,11-dihydro-6,6-dimethyl-8-(4-(4-morpholinyl)-1-piperidinyl)-11-oxo-, Hydrochloride (1:1)
16. Alectinib Monohydrochloride
17. Af-802 (hydrochloride)
18. Agn-pc-09o9bf
19. Ro5424802 (hydrochloride)
20. Chembl3707320
21. Chebi:62268
22. Ch5424802 Hcl
23. Dtxsid10154841
24. Ch 5424802, Alectinib Hcl
25. Bcp09075
26. Ex-a1553
27. Alectinib Hydrochloride [mi]
28. Ch-5424802 Hydrochloride
29. Hy-13011a
30. Mfcd27987893
31. S5232
32. Ccg-264759
33. Cs-3480
34. Sb16516
35. Alectinib Hydrochloride [who-dd]
36. 9-ethyl-6,6-dimethyl-8-(4-morpholin-4-ylpiperidin-1-yl)-11-oxo-5h-benzo[b]carbazole-3-carbonitrile,hydrochloride
37. Ac-29721
38. As-17062
39. Alectinib Hydrochloride [orange Book]
40. D10450
41. Q27104897
42. Ch5424802 Hcl Salt, Alectinib Hcl Salt, Af802 Hcl Salt
43. Ch-5428402 Hcl; Af-802 Hydrochloride; Rg-7853 Hydrochloride; Ro-5424802 Hydrochloride
44. 9-ethyl-6,11-dihydro-6,6-dimethyl-8-[4-(4-morpholinyl)-1-piperidinyl]-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Hydrochloride (1:1)
45. 9-ethyl-6,6-dimethyl-8-(4-morpholin-4-yl-piperidin-1-yl)-11-oxo-6,11-dihydro-5h-benzo[b]carbazole-3-carbonitrile Monohydrochloride Salt
46. 9-ethyl-6,6-dimethyl-8-(4-morpholin-4-ylpiperidin-1-yl)-11-oxo-5h-benzo[b]carbazole-3-carbonitrile;hydrochloride
47. Alectinib Hydrochloride;af-802 Hydrochloride;ch-5424802 Hydrochloride;rg-7853 Hydrochloride;ro-5424802 Hydrochloride
1. Alectinib
2. 1256580-46-7
3. Arq-761
4. Cas 1416163-60-4
5. Cas 1256580-46-7
| Molecular Weight | 519.1 g/mol |
|---|---|
| Molecular Formula | C30H35ClN4O2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 3 |
| Exact Mass | 518.2448541 g/mol |
| Monoisotopic Mass | 518.2448541 g/mol |
| Topological Polar Surface Area | 72.4 Ų |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 867 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Alecensa as monotherapy is indicated for the first-line treatment of adult patients with anaplastic lymphoma kinase (ALK)-positive advanced non-small cell lung cancer (NSCLC).
Alecensa as monotherapy is indicated for the treatment of adult patients with ALKpositive advanced NSCLC previously treated with crizotinib.
L01ED03
