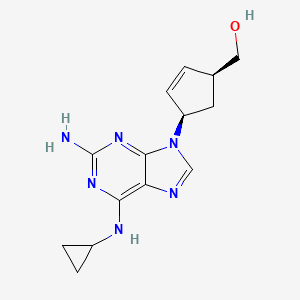

1. ((1r,4r)-4-(2-amino-6-(cyclopropylamino)-9h-purin-9-yl)-cyclopent-2-enyl)methanol
2. ((1r,4s)-4-(2-amino-6-(cyclopropylamino)-9h-purin-9-yl)cyclopent-2-enyl)methanol
3. (+)-abacavir
4. (+)-abacavir Sulfate
5. (+-)-abacavir Sulfate
6. (-)-cis-4-(2-amino-6-(cyclopropylamino)-9h-purin-9-yl)-2-cyclopentene-1-methanol
7. (1r,4r)-abacavir
8. (1r,4s)-abacavir Sulfate
9. (1s,4r)-4-(2-amino-6-(cyclopropylamino)-9h-purin-9-yl)-2-cyclopentene-1-methanol
10. (1s,4r)-4-(2-amino-6-(cyclopropylamino)-9h-purin-9-yl)-2-cyclopentene-1-methanol Succinate (1:1) (salt)
11. 1592u89
12. 2-cyclopentene-1-methanol, 4-(2-amino-6-(cyclopropylamino)-9h-purin-9-yl)-, (1r,4s)-, Sulfate (2:1)
13. 2-cyclopentene-1-methanol, 4-(2-amino-6-(cyclopropylamino)-9h-purin-9-yl)-, (1s,4r)-, Rel-, Sulfate (2:1)
14. 2-cyclopentene-1-methanol, 4-(2-amino-6-(cyclopropylamino)-9h-purin-9-yl)-, Hydrochloride, Hydrate (1:1:1), (1s,4r)-
15. Abacavir Enantiomer
16. Abacavir Hydrochloride Monohydrate
17. Abacavir Succinate
18. Abacavir Sulfate
19. Abacavir Sulfate Racemic
20. Abacavir Sulfate, (+)-
21. Abacavir Sulfate, (+-)-
22. Abacavir Sulfate, (1r,4s)-
23. Abacavir Sulphate
24. Abacavir, (+)-
25. Abacavir, (1r,4s)-
26. Abacavir, Trans-
27. Abacavir, Trans-, (+-)-
28. Abamune
29. Avacavir
30. Drg 0257
31. Drg-0257
32. Drg0257
33. Trans-abacavir
34. Trans-abacavir R,r-
35. Ziagen
1. 136470-78-5
2. Ziagen
3. Abacavir Sulfate
4. Abacavir [inn]
5. 1592u89
6. (1s,4r)-4-[2-amino-6-(cyclopropylamino)-9h-purin-9-yl]-2-cyclopentene-1-methanol
7. Abacavir (inn)
8. Chebi:421707
9. Wr2tip26vs
10. ((1s,4r)-4-(2-amino-6-(cyclopropylamino)-9h-purin-9-yl)cyclopent-2-en-1-yl)methanol
11. Abc
12. [(1s,4r)-4-[2-amino-6-(cyclopropylamino)purin-9-yl]cyclopent-2-en-1-yl]methanol
13. {(1s-cis)-4-[2-amino-6-(cyclopropylamino)-9h-purin-9-yl]cyclopent-2-en-1-yl}methanol
14. Nsc-742406
15. [(1s,4r)-4-[2-amino-6-(cyclopropylamino)purin-9-yl]-1-cyclopent-2-enyl]methanol
16. 2-cyclopentene-1-methanol, 4-[2-amino-6-(cyclopropylamino)-9h-purin-9-yl]-, (1s,4r)-
17. Avacavir
18. 136777-48-5
19. (1s,4r)-4-(2-amino-6-(cyclopropylamino)-9h-purin-9-yl)-2-cyclopentene-1-methanol
20. [(1s,4r)-4-[2-amino-6-(cyclopropylamino)-9h-purin-9-yl]cyclopent-2-en-1-yl]methanol
21. 2-cyclopentene-1-methanol, 4-(2-amino-6-(cyclopropylamino)-9h-purin-9-yl)-, (1s-cis)-
22. (+/-)-abacavir
23. Abacavir [inn:ban]
24. Unii-wr2tip26vs
25. Nsc742406
26. Ziagen (tm)(*succinate Salt*)
27. {(1s,4r)-4-[2-amino-6-(cyclopropylamino)-9h-purin-9-yl]cyclopent-2-en-1-yl}methanol
28. 2-cyclopentene-1-methanol, 4-(2-amino-6-(cyclopropylamino)-9h-purin-9-yl)-, (1s,4r)-
29. Ncgc00164560-01
30. Mfcd00903850
31. Abacavir [mi]
32. Abacavir [vandf]
33. Avacavir [vandf]
34. Abacavir [mart.]
35. Abacavir [who-dd]
36. Epitope Id:137341
37. Abacavir [ema Epar]
38. Chembl1380
39. Schembl38632
40. Mls006010117
41. Dtxsid4046444
42. Gtpl11152
43. Srca-00004
44. Act03218
45. Bcp07728
46. Zinc2015928
47. Bdbm50366816
48. S5215
49. Akos024464970
50. Ac-1299
51. Ccg-267342
52. Cs-1354
53. Db01048
54. Dt-0030
55. Nsc 742406
56. (+/-)-4-[2-amino-6-(cyclopropylamino)-9h-purin-9-yl]-2-cyclopentene-1-methanol
57. (-)-cis-4-(2-amino-6-(cyclopropylamino)-9h-purin-9-yl)-2-cyclopentene-1-methanol
58. Ncgc00164560-02
59. Ncgc00164560-05
60. Ncgc00164560-17
61. Ba166801
62. Hy-17423
63. Smr004701251
64. C07624
65. D07057
66. Ab01566826_01
67. 470a785
68. A807079
69. A905952
70. Q304330
71. J-700136
72. ((1s,4r)-4-(2-amino-6-(cyclopropylamino)-9h-purin-9-yl)cyclopent-2-enyl)methanol
73. (+/-)-cis-4-[2-amino-6-(cyclopropylamino)-9h-purin-9-yl]-2-cyclopentene-1-methanol
74. [(1s,4r)-4-[2-amino-6-(cyclopropylamino)purin-9-yl]cyclopent-2-en-1-yl]methanol;abacavir
| Molecular Weight | 286.33 g/mol |
|---|---|
| Molecular Formula | C14H18N6O |
| XLogP3 | 0.9 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 4 |
| Exact Mass | 286.15420922 g/mol |
| Monoisotopic Mass | 286.15420922 g/mol |
| Topological Polar Surface Area | 102 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 414 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Abacavir |
| PubMed Health | Abacavir (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | Abacavir sulfate is a synthetic carbocyclic nucleoside analogue with inhibitory activity against HIV-1. The chemical name of abacavir sulfate is (1S,cis)-4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]-2-cyclopentene-1-methanol sulfate (salt) (2:1). A... |
| Active Ingredient | Abacavir; Abacavir sulfate |
| Dosage Form | Solution |
| Route | oral |
| Strength | 20mg/ml |
| Market Status | Tentative Approval |
| Company | Aurobindo Pharma; Hetero Labs Unit Iii |
| 2 of 6 | |
|---|---|
| Drug Name | Epzicom |
| PubMed Health | Abacavir/Lamivudine (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Active Ingredient | Abacavir sulfate; lamivudine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 300mg; eq 600mg base |
| Market Status | Prescription |
| Company | Viiv Hlthcare |
| 3 of 6 | |
|---|---|
| Drug Name | Ziagen |
| PubMed Health | Abacavir (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | ZIAGEN is the brand name for abacavir sulfate, a synthetic carbocyclic nucleoside analogue with inhibitory activity against HIV-1. The chemical name of abacavir sulfate is (1S,cis)-4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]-2-cyclopentene-1-metha... |
| Active Ingredient | Abacavir sulfate |
| Dosage Form | Tablet; Solution |
| Route | Oral |
| Strength | eq 20mg base/ml; eq 300mg base |
| Market Status | Prescription |
| Company | Viiv Hlthcare |
| 4 of 6 | |
|---|---|
| Drug Name | Abacavir |
| PubMed Health | Abacavir (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | Abacavir sulfate is a synthetic carbocyclic nucleoside analogue with inhibitory activity against HIV-1. The chemical name of abacavir sulfate is (1S,cis)-4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]-2-cyclopentene-1-methanol sulfate (salt) (2:1). A... |
| Active Ingredient | Abacavir; Abacavir sulfate |
| Dosage Form | Solution |
| Route | oral |
| Strength | 20mg/ml |
| Market Status | Tentative Approval |
| Company | Aurobindo Pharma; Hetero Labs Unit Iii |
| 5 of 6 | |
|---|---|
| Drug Name | Epzicom |
| PubMed Health | Abacavir/Lamivudine (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Active Ingredient | Abacavir sulfate; lamivudine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 300mg; eq 600mg base |
| Market Status | Prescription |
| Company | Viiv Hlthcare |
| 6 of 6 | |
|---|---|
| Drug Name | Ziagen |
| PubMed Health | Abacavir (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | ZIAGEN is the brand name for abacavir sulfate, a synthetic carbocyclic nucleoside analogue with inhibitory activity against HIV-1. The chemical name of abacavir sulfate is (1S,cis)-4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]-2-cyclopentene-1-metha... |
| Active Ingredient | Abacavir sulfate |
| Dosage Form | Tablet; Solution |
| Route | Oral |
| Strength | eq 20mg base/ml; eq 300mg base |
| Market Status | Prescription |
| Company | Viiv Hlthcare |
Abacavir is indicated, in combination with other agents, for treatment of HIV-1 infection. /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1
A unique and potentially fatal hypersensitivity reaction occurs in 2% to 5% of patients receiving abacavir. Symptoms typically occur within the first six weeks of therapy and include fever, rash, nausea, malaise, and respiratory complaints, in various combinations. Symptoms initially may be mild but increase in severity with continued administration. Discontinuation of the medication usually resolves all signs and symptoms, but rechallenge may cause rapid onset of severe reactions, hypotension, and death. Once an abacavir hypersensitivity reaction is suspected or confirmed, it is recommended that the patient never by rechallenged with abacavir.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1359
The major toxicity associated with abacavir therapy is potentially life-threatening hypersensitivity reactions. In clinical studies, hypersensitivity reactions have been reported in approximately 5% of adult and pediatric patients receiving abacavir in conjunction with lamivudine and zidovudine. Fatalities related to hypersensitivity reactions to abacavir have been reported. Manifestations of hypersensitivity usually are apparent within the first 6 weeks of abacavir therapy, but may occur at any time during therapy. Severe hypersensitivity reactions are likely to recur within hours following rechallenge in patients with a prior history of hypersensitivity to the drug, and these reactions may include life-threatening hypotension and death. The most severe hypersensitivity reactions reported to date have been in individuals who were rechallenged with abacavir after a previous hypersensitivity reaction to the drug. There also have been reports of severe or fatal hypersensitivity reactions occurring after abacavir was reintroduced in patients with no identified history of abacavir hypersensitivity or with unrecognized manifestations of hypersensitivity to the drug. Although these patients had discontinued abacavir for reasons unrelated to hypersensitivity (e.g., interruption in drug supply, discontinuance of abacavir during treatment for other medical conditions), some may have had symptoms present before discontinuance of the drug that were consistent with hypersensitivity but were attributed to other medical conditions (e.g., acute onset respiratory disease, gastroenteritis, adverse reactions to other drugs). Most of the hypersensitivity reactions reported following reintroduction of abacavir in these patients were indistinguishable from hypersensitivity reactions associated with abacavir rechallenge (i.e., short time to onset, increased severity of symptoms, poor outcome including death).Hypersensitivity reactions can occur within hours after abacavir is reintroduced; however, in some cases, these reactions occurred days to weeks following reintroduction of the drug.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 619
Lactic acidosis and severe hepatomegaly with steatosis (sometimes fatal) have been reported rarely in patients receiving abacavir and also have been reported in patients receiving dideoxynucleoside reverse transcriptase inhibitors. Most reported cases have involved women; obesity and long-term therapy with a nucleoside reverse transcriptase inhibitor also may be risk factors. Increased serum concentrations of Gamma-glutamyltransferase (GGT, GGPT) have been reported in patients receiving abacavir.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 620
Hypersensitivity reactions reported in patients receiving abacavir are characterized by the appearance of manifestations indicating involvement of multiple organ and body systems; these reactions have occurred in association with anaphylaxis, liver failure, renal failure, hypotension, and death. The most frequent manifestations of hypersensitivity reactions to abacavir include fever, rash, fatigue, GI symptoms such as nausea, vomiting, diarrhea, and abdominal pain, and respiratory symptoms such as pharyngitis, dyspnea, and cough. Other signs and symptoms include malaise, lethargy, myalgia, myolysis, headache, arthralgia, edema, paresthesia, lymphadenopathy, and mucous membrane lesions (e.g., conjunctivitis, mouth ulceration). Respiratory symptoms, including cough, dyspnea, and pharyngitis, have been reported in approximately 20% of patients with hypersensitivity reactions to abacavir. Fatalities have occurred in patients who developed hypersensitivity reactions in which the initial presentation included respiratory symptoms; some patients who experienced fatal hypersensitivity reactions were initially diagnosed as having an acute respiratory disease (pneumonia, bronchitis, flu-like illness). Hypersensitivity reactions can occur without rash; if rash occurs, it usually is maculopapular or urticarial, but may be variable in appearance. Laboratory abnormalities reported in patients experiencing a hypersensitivity reaction to abacavir include lymphopenia and increases in serum concentrations of liver enzymes, creatine kinase (CK, creatine phosphokinase, CPK), or creatinine.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 619
For more Drug Warnings (Complete) data for ABACAVIR SULFATE (17 total), please visit the HSDB record page.
For the treatment of HIV-1 infection, in combination with other antiretroviral agents.
FDA Label
Ziagen is indicated in antiretroviral combination therapy for the treatment of Human Immunodeficiency Virus (HIV) infection in adults, adolescents and children.
The demonstration of the benefit of Ziagen is mainly based on results of studies performed with a twice daily regimen, in treatment-nave adult patients on combination therapy.
Before initiating treatment with abacavir, screening for carriage of the HLA-B*5701 allele should be performed in any HIV-infected patient, irrespective of racial origin. Abacavir should not be used in patients known to carry the HLA-B*5701 allele.
Abacavir is a nucleoside reverse transcriptase inhibitor (NRTI) with activity against Human Immunodeficiency Virus Type 1 (HIV-1). Abacavir is phosphorylated to active metabolites that compete for incorporation into viral DNA. They inhibit the HIV reverse transcriptase enzyme competitively and act as a chain terminator of DNA synthesis. The concentration of drug necessary to effect viral replication by 50 percent (EC50) ranged from 3.7 to 5.8 M (1 M = 0.28 mcg/mL) and 0.07 to 1.0 M against HIV-1IIIB and HIV-1BaL, respectively, and was 0.26 0.18 M against 8 clinical isolates. Abacavir had synergistic activity in cell culture in combination with the nucleoside reverse transcriptase inhibitor (NRTI) zidovudine, the non-nucleoside reverse transcriptase inhibitor (NNRTI) nevirapine, and the protease inhibitor (PI) amprenavir; and additive activity in combination with the NRTIs didanosine, emtricitabine, lamivudine, stavudine, tenofovir, and zalcitabine.
Reverse Transcriptase Inhibitors
Inhibitors of reverse transcriptase (RNA-DIRECTED DNA POLYMERASE), an enzyme that synthesizes DNA on an RNA template. (See all compounds classified as Reverse Transcriptase Inhibitors.)
Anti-HIV Agents
Agents used to treat AIDS and/or stop the spread of the HIV infection. These do not include drugs used to treat symptoms or opportunistic infections associated with AIDS. (See all compounds classified as Anti-HIV Agents.)
J05AF06
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AF - Nucleoside and nucleotide reverse transcriptase inhibitors
J05AF06 - Abacavir
Absorption
Rapid and extensive after oral administration (83% bioavailability, tablet). When a 300 mg tablet is given twice daily to subjects, the peak plasma concentration (Cmax) was 3.0 0.89 mcg/mL and the area under the curve (AUC 0-12 hours) was 6.02 1.73 mcghr/mL.
Route of Elimination
Elimination of abacavir was quantified in a mass balance study following administration of a 600-mg dose of 14C-abacavir: 99% of the radioactivity was recovered, 1.2% was excreted in the urine as abacavir, 30% as the 5-carboxylic acid metabolite, 36% as the 5-glucuronide metabolite, and 15% as unidentified minor metabolites in the urine. Fecal elimination accounted for 16% of the dose. Renal excretion of unchanged abacavir is a minor route of elimination in humans.
Volume of Distribution
0.86 0.15 L/kg [IV administration]
Clearance
0.80 0.24 L/hr/kg [asymptomatic, HIV-1-infected adult patients receiving single (IV dose of 150mg]
Following oral administration of a 600-mg dose of radiolabeled abacavir, 82.2% of the dose is excreted in urine and 16% of the dose is excreted in feces. The 5-carboxylic acid metabolite, 5-glucuronide metabolite, and unchanged abacavir accounted for 30, 36, and 1.2%, respectively, of recovered radioactivity in urine; unidentified minor metabolites accounted for 15% of recovered radioactivity in urine.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 623
It is not known whether abacavir is distributed into human milk; the drug is distributed into milk in rats.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 621
Abacavir crosses the placenta in rats.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 621
The oral bioavailability of abacavir is high with or without food; the CSF-to-plasma AUC ratio is approximately 0.3.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1359
For more Absorption, Distribution and Excretion (Complete) data for ABACAVIR SULFATE (7 total), please visit the HSDB record page.
Hepatic, by alcohol dehydrogenase and glucuronosyltransferase to a 5′-carboxylic acid metabolite and 5′-glucuronide metabolite, respectively. These metabolites have no antiviral activity. Abacavir is not significantly metabolized by cytochrome P450 enzymes.
Abacavir is partially metabolized by alcohol dehydrogenase (to form the 5'-carboxylic acid) and glucuronidation (to form the 5'-glucuronide).
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1359
The metabolic fate of abacavir has not been fully determined, but the drug is metabolized in the liver. Abacavir is metabolized by alcohol dehydrogenase to form the 5-carboxylic acid and by glucuronyltransferase to form the 5-glucuronide; these metabolites do not appear to have any antiviral activity. Any involvement of cytochrome p450 isoenzymes in the metabolism of abacavir is limited.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 623
Intracellularly, abacavir is phosphorylated to abacavir monophosphate by adenosine phosphotransferase; abacavir monophosphate is then converted to carbovir monophosphate in a reaction catalyzed by cytosolic enzymes and then to carbovir triphosphate by cellular kinases. Intracellular (host cell) conversion of abacavir to carbovir triphosphate is necessary for the antiviral activity of the drug. The in vitro intracellular half-life of carbovir triphosphate in CD4+ CEM cells is 3.3 hours.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 623
1.54 0.63 hours
The in vitro intracellular half-life of carbovir triphosphate /SRP: a metabolite of abacavir sulfate,/ in CD4+ CEM cells is 3.3 hours.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 623
The plasma elimination half-life of abacavir following a single oral dose (given as abacavir sulfate) is about 1.5 hours. In HIV-infected children 3 months to 13 years of age who received 8 mg/kg of abacavir every 12 hours (given as an oral solution containing abacavir sulfate), steady-state plasma elimination half-life averaged 1.3 hours and was essentially the same as that reported after a single dose. Following oral administration of a single 300-mg dose of abacavir to an individual with renal failure (glomerular filtration rate less than 10 mL/minute) undergoing peritoneal dialysis, the plasma elimination half-life of the drug was 1.33 hours.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 623
Abacavir is a carbocyclic synthetic nucleoside analogue and an antiviral agent. Intracellularly, abacavir is converted by cellular enzymes to the active metabolite carbovir triphosphate, an analogue of deoxyguanosine-5'-triphosphate (dGTP). Carbovir triphosphate inhibits the activity of HIV-1 reverse transcriptase (RT) both by competing with the natural substrate dGTP and by its incorporation into viral DNA. Viral DNA growth is terminated because the incorporated nucleotide lacks a 3'-OH group, which is needed to form the 5 to 3 phosphodiester linkage essential for DNA chain elongation.
Like dideoxynucleoside reverse transcriptase inhibitors (e.g., didanosine, lamivudine, stavudine, zalcitabine, zidovudine), the antiviral activity of abacavir appears to depend on intracellular conversion of the drug to a 5-triphosphate metabolite; thus, carbovir triphosphate (carbocyclic guanosine triphosphate) and not unchanged abacavir appears to be the pharmacologically active form of the drug. Substantial differences exist in the rates at which human cells phosphorylate various nucleoside antiviral agents and in the enzymatic pathways involved.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 621
Enzymatic conversion of abacavir to carbovir triphosphate appears to be complex and involves certain steps and enzymes that differ from those involved in the enzymatic conversion of dideoxynucleoside reverse transcriptase inhibitors. Abacavir is phosphorylated by adenosine phosphotransferase to abacavir monophosphate, which is converted to carbovir monophosphate by a cytosolic enzyme. Subsequently, carbovir monophosphate is phosphorylated by cellular kinases to carbovir triphosphate. Abacavir is not a substrate for enzymes (i.e., thymidine kinase, deoxycytidine kinase, adenosine kinase, mitochondrial deoxyguanosine kinase) known to phosphorylate other nucleoside analogs. Because phosphorylation of abacavir depends on cellular rather than viral enzymes, conversion of the drug to the active triphosphate derivative occurs in both virus-infected and uninfected cells. Carbovir triphosphate is a structural analog of deoxyguanosine-5-triphosphate (dGTP), the usual substrate for viral RNA-directed DNA polymerase. Although other mechanisms may be involved in the antiretroviral activity of the drug, carbovir triphosphate appears to compete with deoxyguanosine-5-triphosphate for viral RNA-directed DNA polymerase and incorporation into viral DNA. Following incorporation of carbovir triphosphate into the viral DNA chain instead of deoxyguanosine-5-triphosphate, DNA synthesis is prematurely terminated because the absence of the 3-hydroxy group on the drug prevents further 5 to 3 phosphodiester linkages.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 621
The complete mechanism(s) of antiviral activity of abacavir has not been fully elucidated. Following conversion to a pharmacologically active metabolite, abacavir apparently inhibits replication of retroviruses, including human immunodeficiency virus type 1 (HIV-1) and type 2 (HIV-2), by interfering with viral RNA-directed DNA polymerase (reverse transcriptase). The drug, therefore, exerts a virustatic effect against retroviruses by acting as a reverse transcriptase inhibitor.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 621