

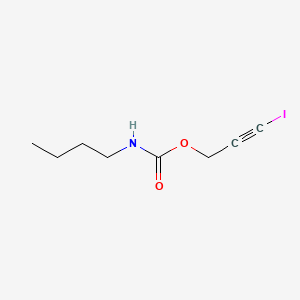

1. 3-iodo-2-propynylbutylcarbamate
2. 3-ipbc
3. 3-iodo-2-propynyl-butylcarbamate
4. Iodopropynyl Butylcarbamate
1. 55406-53-6
2. Iodopropynyl Butylcarbamate
3. Ipbc
4. Woodlife
5. Troysan Kk-108a
6. Caswell No. 501a
7. Butyl-3-iodo-2-propynylcarbamate
8. 3-iodo-2-propynyl Butyl Carbamate
9. 3-iodoprop-2-yn-1-yl N-butylcarbamate
10. 603p14dheb
11. 3-iodo-2-propyn-1-yl N-butylcarbamate
12. Carbamic Acid, Butyl-3-iodo-2-propynyl Ester
13. 3-iodoprop-2-ynyl Butylcarbamate
14. 3-ipbc
15. 3-iodo-2-propynyl-butylcarbamate
16. Refchem:502679
17. Iodopropynyl Butyl Carbamate
18. 259-627-5
19. 3-iodo-2-propynylbutylcarbamate
20. 3-iodoprop-2-yn-1-yl Butylcarbamate
21. Iodocarb
22. 3-iodo-2-propynyl N-butylcarbamate
23. 3-iodoprop-2-ynyl N-butylcarbamate
24. Carbamic Acid, Butyl-, 3-iodo-2-propynyl Ester
25. 1-iodoprop-1-yn-3-yl N-n-butylcarbamate
26. 3-iodo-2-propynyl-n-butylcarbamate
27. Mfcd00072438
28. C8h12ino2
29. Dtxsid0028038
30. Chebi:83279
31. 85045-09-6
32. Dtxcid908038
33. Cas-55406-53-6
34. 3-iodo-2-propynyl N-butylcarbamate-d9
35. Hsdb 7314
36. Einecs 259-627-5
37. Epa Pesticide Chemical Code 107801
38. Brn 2248232
39. Iodocarbe
40. Unii-603p14dheb
41. 3-iodo-2-propynyl-n-butyl Carbamate
42. 1246815-08-6
43. Iodocarb 100 Microg/ml In Acetonitrile
44. 3-iodo-2-propynylbutyl Carbamate
45. Ipbc [mi]
46. Schembl114369
47. Chembl1893913
48. 3-iodo-2-propynyl Butylcarbamate #
49. 3-iodoprop-2-yn-1-ylbutylcarbamate
50. Tox21_201864
51. Tox21_301117
52. Akos015905567
53. Cs-w010051
54. Fi16109
55. Gs-3240
56. Ncgc00164376-01
57. Ncgc00164376-02
58. Ncgc00164376-03
59. Ncgc00164376-04
60. Ncgc00164376-05
61. Ncgc00255017-01
62. Ncgc00259413-01
63. Sy052464
64. 3-iodo-2-propynyl N-butylcarbamate, 97%
65. Iodopropynyl Butylcarbamate [vandf]
66. 3-iodo-2-propynyl Butyl Carbbamate
67. Db-262370
68. I0666
69. Iodopropynyl Butyl Carbamate [mart.]
70. Ns00000275
71. N-butylcarbamic Acid 3-iodo-2-propynyl Ester
72. 06i536
73. 3-iodo-2-propynylbutylcarbamate [hsdb]
74. F050829
75. Q2928998
76. Carbamic Acid, N-butyl-, 3-iodo-2-propyn-1-yl Ester
77. 3-iodo-2-propynyl N-butylcarbamate, Analytical Standard
| Molecular Weight | 281.09 g/mol |
|---|---|
| Molecular Formula | C8H12INO2 |
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 5 |
| Exact Mass | Da |
| Monoisotopic Mass | Da |
| Topological Polar Surface Area | 38.3 |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 192 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
...IPBC was administered orally in 0.5% carboxymethylcellulose to groups of male and female Crl:CD/BR rats in the following manner: Groups of 5 male and 5 female rats (groups A and B) received either a single oral high dose of radiolabelled IPBC (125 mg/kg) or a repeated low oral dose of non-radiolabelled IPBC followed by a single radiolabelled dose (20 mg/kg). Separate groups of rats (9/sex/group, groups C and D) received single oral doses (20 and 125 mg/kg) of radiolabelled IPBC, and 3 rats/sex were sacrificed at 2, 4, and 120 hours post-dose for determination of tissue distribution of radioactivity. Urine, feces and expired air were collected at 24 hr intervals for groups A and B, while urine and feces were collected from groups C and D. Absorption of test chemical at the low and high dose was between 80-90% for all dose groups, as suggested by excretion data showing the majority of a dose eliminated through urine or exhaled air. Excretion of IPBC-derived radioactivity was mainly via the urine, with between 50-70% of an administered dose excreted by this route at 168 hours post-dose. Feces was a minor route of excretion in all dose groups (4-7% of the administered dose), while radiolabelled CO2 constituted between 18-24% of the administered dose. Repeated low oral dosing or a single high oral dose appeared to result in a decrease in the percentage of radioactivity excreted as 14-CO2 compared to a single low dose.
USEPA/Office of Pesticide Programs; Reregistration Eligibility Decision Document - 3-Iodo-2-propynyl butylcarbamate(IPBC). EPA 738-R-97-003 March 1997. Available from, as of January 31, 2005: https://www.epa.gov/pesticides/reregistration/status.htm
...IPBC undergoes reductive dehalogenation followed by dealkylation to form the URM-9 and URM-10 metabolites. In addition, de-carboxylation following reductive dehalogenation yields carbon dioxide. Various other metabolites formed from dehalogenation are glucuronidated and constitute minor metabolites of IPBC.
USEPA/Office of Pesticide Programs; Reregistration Eligibility Decision Document - 3-Iodo-2-propynyl butylcarbamate(IPBC). EPA 738-R-97-003 March 1997. Available from, as of January 31, 2005: https://www.epa.gov/pesticides/reregistration/status.htm
The carbamates are hydrolyzed enzymatically by the liver; degradation products are excreted by the kidneys and the liver. (L793)
