

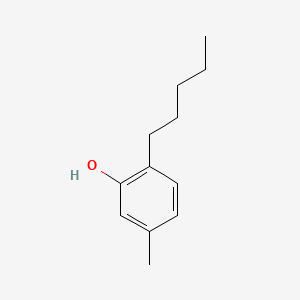

1. 5-methyl-2-pentylphenol
2. 1300-94-3
3. 5-methyl-2-n-pentylphenol
4. 6-pentyl-m-cresol
5. 6-amyl-m-cresol
6. 6-n-amyl-m-cresol
7. 6-n-pentyl-m-cresol
8. Amylmetacresolum
9. Phenol, 5-methyl-2-pentyl-
10. Amyl Metacresol
11. 2-amyl-5-methylphenol
12. Chebi:48213
13. 05w904p57f
14. Ncgc00180998-01
15. Amilmetacresol
16. Amilmetacresol [spanish]
17. Amylmetacresolum [latin]
18. Amylmetacresol [inn:ban]
19. Brn 2440952
20. M-cresol, 6-pentyl-
21. Einecs 215-094-0
22. Unii-05w904p57f
23. Amylmetacresol [inn]
24. Dsstox_cid_26791
25. Dsstox_rid_81908
26. Dsstox_gsid_46791
27. 3-06-00-02005 (beilstein Handbook Reference)
28. Amylmetacresol [vandf]
29. Schembl302197
30. Amylmetacresol [mart.]
31. Amyl Metacresol [vandf]
32. Amylmetacresol [who-dd]
33. Chembl1512677
34. Dtxsid8046791
35. 6-n-amyl-m-cresol [mi]
36. Baa30094
37. Zinc2039651
38. Amylmetacresol [ep Impurity]
39. Tox21_112651
40. Amylmetacresol [ep Monograph]
41. Mfcd00127710
42. S6173
43. Stl453583
44. Akos015912095
45. Db13908
46. 5-methyl-2-n-pentylphenol, Aldrichcpr
47. Ds-15450
48. Cas-1300-94-3
49. Hy-121527
50. Cs-0082471
51. Ft-0620948
52. Amylmetacresol 100 Microg/ml In Acetonitrile
53. A888790
54. Sr-01000944860
55. Q1946346
56. Sr-01000944860-1
57. W-108357
| Molecular Weight | 178.27 g/mol |
|---|---|
| Molecular Formula | C12H18O |
| XLogP3 | 4.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 4 |
| Exact Mass | 178.135765193 g/mol |
| Monoisotopic Mass | 178.135765193 g/mol |
| Topological Polar Surface Area | 20.2 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 133 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Sore throat, minor mouth and throat infections,,.
The mixture of amylmetacresol throat lozenge medications markedly reduces the infectivity of certain infectious viruses in the throat and in cough droplets, thus reducing opportunities for person-to-person transmission. In addition, it relieves symptoms of sore throat/irritation of the throat,. Amylmetacresol is thought to inhibit the inflammatory and pain mediators that are involved in the inflammation of the mouth and throat mucous membranes, as well as the sore throat.
Absorption
Rapidly absorbed and eliminated.
The mechanism of virucidal action is not fully elucidated, however it is suggested that denaturation of external protein spikes, a pH-induced rearrangement of the tertiary structure of attachment proteins, or a selective effect on viral lipid membranes/proteinlipid interaction is responsible for this action. Amylmetacresol is an antibacterial and antiviral agent, and blocks voltage-gated Na channels in a local anesthetic-like manner.